Label: ALPRAZOLAM tablet, orally disintegrating
- NDC Code(s): 49884-110-74, 49884-111-74, 49884-213-74, 49884-214-74
- Packager: Par Pharmaceutical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ALPRAZOLAM ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ALPRAZOLAM ORALLY DISINTEGRATING TABLETS.
ALPRAZOLAM orally disintegrating tablets, C-IV
Initial U.S. Approval: 2005WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND
WITHDRAWAL REACTIONS
See full prescribing information for complete boxed warning.
- The use of benzodiazepines, including alprazolam orally disintegrating tablets, exposes users to risks of abuse, misuse, and addiction. Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (5.1, 7.1).
-
The use of benzodiazepines, including alprazolam orally disintegrating tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Before prescribing alprazolam orally disintegrating tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (5.2).
- Abrupt discontinuation or rapid dosage reduction of alprazolam orally disintegrating tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam orally disintegrating tablets or reduce the dosage (2.3, 5.3).
RECENT MAJOR CHANGES
Warnings and Precautions (5.8) 08/2022
INDICATIONS AND USAGE
Alprazolam orally disintegrating tablets are a benzodiazepine indicated for:
- The treatment of generalized anxiety disorder (1.1). The efficacy of alprazolam was demonstrated in 5 short-term, placebo-controlled trials (14).
- The treatment of panic disorder, with or without agoraphobia (1.2). The efficacy of alprazolam in the treatment of panic disorder was established in 2 short-term, placebo-controlled trials (14).
DOSAGE AND ADMINISTRATION
Indication
Recommended Dose
Anxiety Disorder (2.1)
Initial: 0.25 mg to 0.5 mg given three times daily. Maximum: 4 mg per day given in divided doses.
Panic Disorder (2.2)
Initial: 0.5 mg given three times daily. Maximum: Doses up to 10 mg per day may be required to achieve a successful response.
- With dry hands, place the tablet on top of the tongue where it will disintegrate and be swallowed with saliva (2.5).
- Depending on response, the dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days (2.1, 2.2).
- Use the lowest possible effective dose.
- Periodically reassess the need for continued treatment (2.1).
- In general, benzodiazepines should be prescribed for short periods (2). Discontinuation of treatment or dose reduction should be gradual and under close physician supervision. Decrease the dosage by no more than 0.5 mg per day every 3 days. Some patients may require an even slower dosage reduction (2.1,2.2).
- Dosing in elderly: the starting dose is 0.25 mg, given two or three times daily (2.4).
- Severe hepatic impairment: the starting dose is 0.25 mg, given two or three times daily (2.4).
DOSAGE FORMS AND STRENGTHS
0.25 mg, 0.5 mg, 1 mg, or 2 mg scored orally disintegrating tablets USP (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Suicide: As with other psychotropic medications, use precautions with respect to administration of the drug and size of the prescription, especially in patients who are severely depressed or in patients where there is reason to expect concealed suicidal ideation or plans (5.4).
-
Status Epilepticus and Seizure: can occur during discontinuation of alprazolam (5.5).
- CNS Depression and Impaired Cognitive and Motor Performance: caution patients against engaging in hazardous occupations or activities requiring complete mental alertness, until they are reasonably certain that alprazolam treatment does not affect them adversely. Caution patients about the use of alcohol and other CNS depressant drugs during treatment with alprazolam (5.7).
- Neonatal Sedation and Withdrawal Syndrome: Alprazolam use during pregnancy can result in neonatal sedation and/or neonatal withdrawal (5.8, 8.1).
- Interdose anxiety symptoms: can occur at prescribed maintenance doses. Consider dividing the daily dose into more frequent administrations (5.10).
- Patients with Concomitant Illness: In the elderly or debilitated patients, the smallest effective dose is recommended to preclude the development of ataxia or oversedation. There have been rare reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with alprazolam (5.12).
ADVERSE REACTIONS
- Anxiety Disorder: The most common adverse reactions (greater than or equal to 5% and ~twice the rate of placebo) were sedation, and hypotension.
- Panic Disorder: The most common adverse reactions included sedation, impaired coordination, dysarthria, and increased libido (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Alprazolam produces additive CNS depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistaminics, alcohol and other drugs that produce CNS depression (7.1).
- The formulation requires an acidic environment to dissolve; therefore, drugs or diseases that cause dry mouth or raise stomach pH may slow disintegration or dissolution, resulting in decreased absorption (7.2).
- Drugs which inhibit the hydroxylation catalyzed by cytochrome P450 3A (CYP3A) metabolic pathway can decrease the clearance of alprazolam and increase the serum concentration (7.4).
USE IN SPECIFIC POPULATIONS
- Teratogenic Effects: Pregnancy Category D. Alprazolam can cause fetal harm (8.1).
- Nonteratogenic Effects: A neonate born to a mother who is treated with alprazolam may be at risk for withdrawal, flaccidity, and respiratory problems (8.1).
- Nursing Mothers: Chronic administration of diazepam to nursing mothers has been reported to cause their infants to become lethargic and to lose weight (8.3).
- Geriatric Use: The elderly exhibit higher plasma concentrations due to reduced clearance, compared with a younger population receiving the same doses (8.5).
- Pediatric Use: Safety and effectiveness of alprazolam in individuals below 18 years of age have not been established (8.4).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and
DEPENDENCE AND WITHDRAWAL REACTIONS1 INDICATIONS AND USAGE
1.1 Generalized Anxiety Disorder
1.2 Panic Disorder
2 DOSAGE AND ADMINISTRATION
2.1 Generalized Anxiety Disorder
2.2 Panic Disorder
2.3 Discontinuation or Dosage Reduction of Alprazolam Orally Disintegrating Tablets
2.4 Dosing in Special Populations
2.5 Instructions to be Given to Patients for Use/Handling Alprazolam Orally Disintegrating Tablets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 Abuse, Misuse, and Addiction
5.3 Dependence and Withdrawal Reactions
5.4 Suicide and Overdose
5.5 Status Epilepticus
5.6 CNS Depression and Impaired Performance
5.7 Mania
5.8 Neonatal Sedation and Withdrawal Syndrome
5.9 Alprazolam Interaction with Drugs that Inhibit Metabolism via Cytochrome P450 3A
5.10 Interdose Symptoms
5.11 Uricosuric Effect
5.12 Use in Patients with Concomitant Illness
5.13 Risks in Patients with Phenylketonuria
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Use with Other CNS Depressants
7.2 Drugs Effecting Salivary Flow and Stomach pH
7.3 Use with Imipramine and Desipramine
7.4 Drugs that Inhibit Alprazolam Metabolism via Cytochrome P450 3A
7.5 Drugs Demonstrated to be CYP3A Inhibitors of Possible Clinical Significance on the Basis of Clinical Studies Involving Alprazolam
7.6 Drugs and Other Substances Demonstrated to be CYP3A Inhibitors on the Basis of Clinical Studies Involving Benzodiazepines Metabolized Similarly to Alprazolam or on the Basis of In Vitro Studies with Alprazolam or Other Benzodiazepines
7.7 Inducers of CYP3A
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
11.1 Alprazolam Orally Disintegrating Tablets, USP
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Anxiety Disorders
14.2 Panic Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and
DEPENDENCE AND WITHDRAWAL REACTIONS- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
-
The use of benzodiazepines, including Alprazolam orally disintegrating tablets, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Alprazolam orally disintegrating tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction [see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines, including Alprazolam orally disintegrating tablets, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of Alprazolam orally disintegrating tablets after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Alprazolam orally disintegrating tablets or reduce the dosage [see Dosage and Administration (2.3) and Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGE
1.1 Generalized Anxiety Disorder
Alprazolam orally disintegrating tablets, USP are indicated for the treatment of generalized anxiety disorder.
The efficacy of alprazolam in the treatment of generalized anxiety disorder was demonstrated in 5 short-term, placebo-controlled trials [see Clinical Studies (14.1)].
1.2 Panic Disorder
Alprazolam orally disintegrating tablets, USP are also indicated for the treatment of panic disorder, with or without agoraphobia.
The efficacy of alprazolam in the treatment of panic disorder was established in 2 short-term, placebo-controlled trials [see Clinical Studies (14.2)].
Demonstrations of the effectiveness of alprazolam by systematic clinical study are limited to 4 months in duration for generalized anxiety disorder and 4 to 10 weeks duration for panic disorder; however, patients with panic disorder have been treated on an open basis for up to 8 months without apparent loss of benefit. The physician should periodically reassess the usefulness of the drug for the individual patient.
-
2 DOSAGE AND ADMINISTRATION
Dosage should be individualized for maximum beneficial effect. While the usual daily dosages given below will meet the needs of most patients, there will be some who require doses greater than 4 mg per day. In such cases, the dosage should be increased cautiously to avoid adverse reactions. In general, benzodiazepines should be prescribed for short periods. Reevaluate the need for continued therapy before extending the treatment period.
2.1 Generalized Anxiety Disorder
Initiate treatment with a dose of 0.25 mg to 0.5 mg three times daily. The dose may be increased to achieve a maximum therapeutic effect, at intervals of 3 to 4 days, to a maximum daily dose of 4 mg, given in divided doses. Use the lowest possible effective dose, and periodically reassess the need for continued treatment. The risk of dependence can increase with dose and duration of treatment.
2.2 Panic Disorder
The successful treatment of many panic disorder patients has required the use of alprazolam at doses greater than 4 mg daily. In controlled trials conducted to establish the efficacy of alprazolam in panic disorder, doses in the range of 1 mg to 10 mg daily were used. The mean dosage employed was approximately 5 mg to 6 mg daily. Among the approximately 1700 patients participating in the panic disorder development program, about 300 received alprazolam in dosages of greater than 7 mg per day, including approximately 100 patients who received maximum dosages of greater than 9 mg per day. Occasional patients required as much as 10 mg a day to achieve a successful response.
Dose Titration
Initiate treatment with a dose of 0.5 mg three times daily. Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg per day. Slower titration to the dose levels greater than 4 mg per day may be advisable to allow full expression of the pharmacodynamic effect of alprazolam. To lessen the possibility of interdose symptoms, the times of administration should be distributed as evenly as possible throughout the waking hours, (i.e., administered three or four times daily).
Generally, therapy should be initiated at a low dose to minimize the risk of adverse responses in patients especially sensitive to the drug. The dose should be advanced until an acceptable therapeutic response (i.e., a substantial reduction in or total elimination of panic attacks) is achieved, intolerance occurs, or the maximum recommended dose is attained.
Dose Maintenance
For patients receiving doses greater than 4 mg per day, periodically reassess treatment and consider a reduction of dosage. In a controlled postmarketing dose-response study, patients treated with doses of alprazolam greater than 4 mg per day for 3 months were able to taper to 50% of their total daily maintenance dose without apparent loss of clinical benefit. Because of the danger of withdrawal, avoid abrupt discontinuation of treatment [see Warnings and Precautions (5.3), Drug Abuse and Dependence (9.3)].
The necessary duration of treatment for panic disorder patients responding to alprazolam is unknown. After a period of extended freedom from attacks, a carefully supervised tapered discontinuation may be attempted, but there is evidence that this may often be difficult to accomplish without recurrence of symptoms and/or the manifestation of withdrawal phenomena.
2.3 Discontinuation or Dosage Reduction of Alprazolam Orally Disintegrating Tablets
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Alprazolam orally disintegrating tablets or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly [see Warnings and Precautions (5.3) and Drug Abuse and Dependence (9.3)].
In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, there was no difference between the groups in the proportion of patients who tapered and completely discontinued treatment with alprazolam; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome. Reduce the dose by no more than 0.5 mg every 3 days. Some patients may benefit from an even more gradual discontinuation. Some patients may prove resistant to all discontinuation regimens.
2.4 Dosing in Special Populations
In elderly patients, in patients with advanced liver disease, or in patients with debilitating disease (e.g., severe pulmonary disease), the usual starting dose is 0.25 mg, given two or three times daily. This may be gradually increased if needed and tolerated. The elderly may be especially sensitive to the effects of benzodiazepines. If adverse reactions occur at the recommended starting dose, the dose may be lowered.
2.5 Instructions to be Given to Patients for Use/Handling Alprazolam Orally Disintegrating Tablets
Just prior to administration, with dry hands, remove the tablet from the blister. Immediately place the alprazolam orally disintegrating tablet on top of the tongue where it will disintegrate and be swallowed with saliva. Administration with liquid is not necessary.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Alprazolam orally disintegrating tablets are contraindicated in patients with acute narrow angle glaucoma. Alprazolam orally disintegrating tablets can exacerbate narrow angle closure. Alprazolam orally disintegrating tablets may be used in patients with open angle glaucoma who are receiving appropriate therapy.
Alprazolam orally disintegrating tablets are contraindicated in patients treated with potent CYP3A4 inhibitors (e.g., ketoconazole and itraconazole), because these medications significantly impair the oxidative metabolism mediated by cytochrome P450 3A (CYP3A) and can increase alprazolam exposures [see Clinical Pharmacology (12.3), Warnings and Precautions (5.9), and Drug Interactions (7.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including alprazolam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe alprazolam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of alprazolam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking alprazolam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when alprazolam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined [see Drug Interactions (7.1)].
5.2 Abuse, Misuse, and Addiction
The use of benzodiazepines, including Alprazolam orally disintegrating tablets, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death [see Drug Abuse and Dependence (9.2)].
Before prescribing Alprazolam orally disintegrating tablets and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of alprazolam orally disintegrating tablets, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of alprazolam orally disintegrating tablets along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
5.3 Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Alprazolam orally disintegrating tablets or reduce the dosage (a patient-specific plan should be used to taper the dose) [see Dosage and Administration (2.3)].
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including Alprazolam orally disintegrating tablets, lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of Alprazolam orally disintegrating tablets after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) [see Drug Abuse and Dependence (9.3)].
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months [see Drug Abuse and Dependence (9.3)].
Certain adverse clinical events, some life-threatening, are a direct consequence of physical dependence to alprazolam. These include a spectrum of withdrawal symptoms; the most important is seizure [see Drug Abuse and Dependence (9.3)]. Spontaneous reporting system data suggest that the risk of dependence and its severity appear to be greater in patients treated with doses greater than 4 mg per day and for long periods (more than 12 weeks). However, in a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (3 months compared to 6 months) had no effect on the ability of patients to taper to zero dose. In contrast, patients treated with doses of alprazolam greater than 4 mg per day had more difficulty tapering to zero dose than those treated with less than 4 mg per day.
The importance of dose and the risks of Alprazolam as a treatment for panic disorder
Because the management of panic disorder often requires the use of average daily doses of alprazolam above 4 mg, the risk of dependence among panic disorder patients may be higher than that among those treated for less severe anxiety. Experience in randomized placebo-controlled discontinuation studies of patients with panic disorder showed a high rate of rebound and withdrawal symptoms in patients treated with alprazolam compared to placebo-treated patients.
Relapse or return of illness was defined as a return of symptoms characteristic of panic disorder (primarily panic attacks) to levels approximately equal to those seen at baseline before active treatment was initiated. Rebound refers to a return of symptoms of panic disorder to a level substantially greater in frequency, or more severe in intensity than seen at baseline. Withdrawal symptoms were identified as those which were generally not characteristic of panic disorder and which occurred for the first time more frequently during discontinuation than at baseline.
In a controlled clinical trial in which 63 patients were randomized to alprazolam and where withdrawal symptoms were specifically sought, the following were identified as symptoms of withdrawal: heightened sensory perception, impaired concentration, dysosmia, clouded sensorium, paresthesias, muscle cramps, muscle twitch, diarrhea, blurred vision, appetite decrease, and weight loss. Other symptoms, such as anxiety and insomnia, were frequently seen during discontinuation, but it could not be determined if they were due to return of illness, rebound, or withdrawal.
In two controlled trials of 6 to 8 weeks duration where the ability of patients to discontinue medication was measured, 71% to 93% of patients treated with alprazolam tapered completely off therapy compared to 89% to 96% of placebo-treated patients. In a controlled postmarketing discontinuation study of panic disorder patients, the duration of treatment (3 months compared to 6 months) had no effect on the ability of patients to taper to zero dose.
Seizures attributable to alprazolam were seen after drug discontinuance or dose reduction in 8 of 1980 patients with panic disorder or in patients participating in clinical trials where doses of alprazolam greater than 4 mg/day for over 3 months were permitted. Five of these cases clearly occurred during abrupt dose reduction, or discontinuation from daily doses of 2 mg to 10 mg. Three cases occurred in situations where there was not a clear relationship to abrupt dose reduction or discontinuation. In one instance, seizure occurred after discontinuation from a single dose of 1 mg after tapering at a rate of 1 mg every 3 days from 6 mg daily. In two other instances, the relationship to taper is indeterminate; in both of these cases the patients had been receiving doses of 3 mg daily prior to seizure. The duration of use in the above 8 cases ranged from 4 to 22 weeks. There have been occasional voluntary reports of patients developing seizures while apparently tapering gradually from alprazolam. The risk of seizure seems to be greatest 24 to 72 hours after discontinuation [see Dosage and Administration (2)].
To discontinue treatment in patients taking alprazolam, the dosage should be reduced gradually. Decrease the daily dosage of alprazolam by no more than 0.5 mg every three days [see Dosage and Administration (2.3)]. Some patients may benefit from an even slower dosage reduction. In a controlled postmarketing discontinuation study of panic disorder patients which compared this recommended taper schedule with a slower taper schedule, no difference was observed between the groups in the proportion of patients who tapered to zero dose; however, the slower schedule was associated with a reduction in symptoms associated with a withdrawal syndrome.
5.4 Suicide and Overdose
As with other psychotropic medications, the usual precautions with respect to administration of the drug and size of the prescription are indicated for severely depressed patients or those in whom there is reason to expect concealed suicidal ideation or plans. Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients.
5.5 Status Epilepticus
Withdrawal seizures have been reported in association with the discontinuation of alprazolam. In most cases, only a single seizure was reported; however, multiple seizures and status epilepticus were reported as well.
5.6 CNS Depression and Impaired Performance
Because alprazolam has CNS depressant effects and has the potential to impair judgment, cognition, and motor performance, caution patients against engaging in hazardous occupations or activities requiring complete mental alertness such as operating machinery or driving a motor vehicle, until they are reasonably certain that alprazolam treatment does not affect them adversely. Caution patients about the simultaneous ingestion of alcohol and other CNS depressant drugs during treatment with alprazolam.
5.7 Mania
Episodes of hypomania and mania have been reported in association with the use of alprazolam in patients with depression.
5.8 Neonatal Sedation and Withdrawal Syndrome
Use of alprazolam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate [see Use in Specific Populations (8.1)]. Monitor neonates exposed to Alprazolam orally disintegrating tablets during pregnancy or labor for signs of sedation and monitor neonates exposed to Alprazolam orally disintegrating tablets during pregnancy for signs of withdrawal; manage these neonates accordingly.
5.9 Alprazolam Interaction with Drugs that Inhibit Metabolism via Cytochrome P450 3A
The initial step in alprazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP3A). Drugs that inhibit this metabolic pathway may have a profound effect on the clearance of alprazolam. Consequently, alprazolam should be avoided in patients receiving potent inhibitors of CYP3A. With drugs inhibiting CYP3A to a lesser but still significant degree, alprazolam should be used only with caution and consideration of appropriate dosage reduction. For some drugs, an interaction with alprazolam has been quantified with clinical data; for other drugs, interactions are predicted from in vitro data and/or experience with similar drugs in the same pharmacologic class.
The following are examples of drugs known to inhibit the metabolism of alprazolam and/or related benzodiazepines, presumably through inhibition of CYP3A.
Potent CYP3A Inhibitors
Azole antifungal agents— Ketoconazole and itraconazole are potent CYP3A inhibitors and have been shown in vivo to increase plasma alprazolam concentrations 3.98 fold and 2.70 fold, respectively. The coadministration of alprazolam with these agents is not recommended. Other azole-type antifungal agents should also be considered potent CYP3A inhibitors and the coadministration of alprazolam with them is not recommended [see CONTRAINDICATIONS (4)].
Drugs demonstrated to be CYP3A inhibitors on the basis of clinical studies involving alprazolam
Consider dose reduction of alprazolam during coadministration with the following drugs:
- Nefazodone — Coadministration of nefazodone increased alprazolam concentration two-fold.
- Fluvoxamine — Coadministration of fluvoxamine approximately doubled the maximum plasma concentration of alprazolam, decreased clearance by 49%, increased half-life by 71%, and decreased measured psychomotor performance.
- Cimetidine — Coadministration of cimetidine increased the maximum plasma concentration of alprazolam by 86%, decreased clearance by 42%, and increased half-life by 16%.
Other drugs possibly affecting alprazolam metabolism
Other drugs possibly affect alprazolam metabolism by inhibition of CYP3A [see Drug Interactions (7.6)].
5.10 Interdose Symptoms
Early morning anxiety and emergence of anxiety symptoms between doses of alprazolam have been reported in patients with panic disorder taking prescribed maintenance doses of alprazolam. These symptoms may reflect the development of tolerance or a time interval between doses which is longer than the duration of clinical action of the administered dose. In either case, it is presumed that the prescribed dose is not sufficient to maintain plasma levels above those needed to prevent relapse, rebound or withdrawal symptoms over the entire course of the interdosing interval. In these situations, it is recommended that the same total daily dose be given divided as more frequent administrations [see Dosage and Administration (2)].
5.11 Uricosuric Effect
Alprazolam has a weak uricosuric effect. Although other medications with weak uricosuric effect have been reported to cause acute renal failure, there have been no reported instances of acute renal failure attributable to therapy with alprazolam.
5.12 Use in Patients with Concomitant Illness
It is recommended that the dosage be limited to the smallest effective dose to preclude the development of ataxia or oversedation which may be a particular problem in elderly or debilitated patients [see Dosage and Administration (2)]. The usual precautions in treating patients with impaired renal, hepatic or pulmonary function should be observed. There have been rare reports of death in patients with severe pulmonary disease shortly after the initiation of treatment with alprazolam. A decreased systemic alprazolam elimination rate (e.g., increased plasma half-life) has been observed in both alcoholic liver disease patients and obese patients receiving alprazolam [see Clinical Pharmacology (12)].
5.13 Risks in Patients with Phenylketonuria
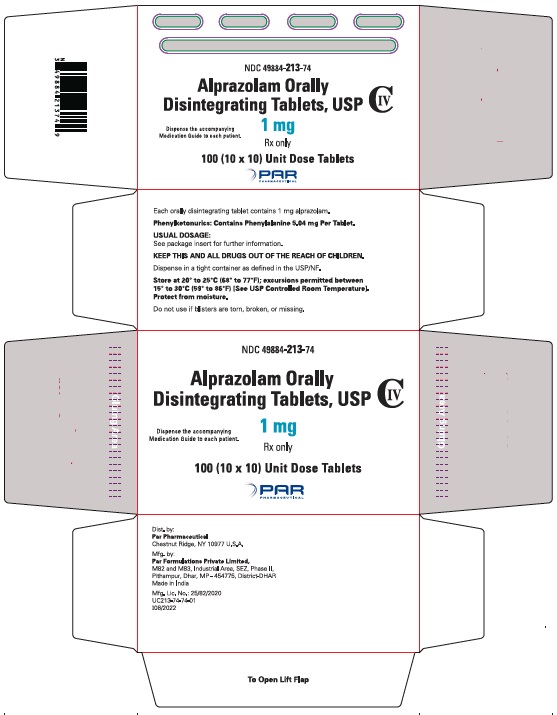
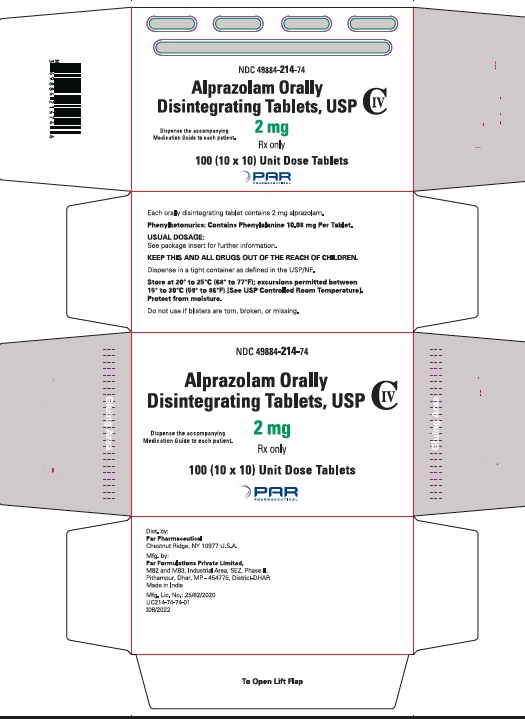
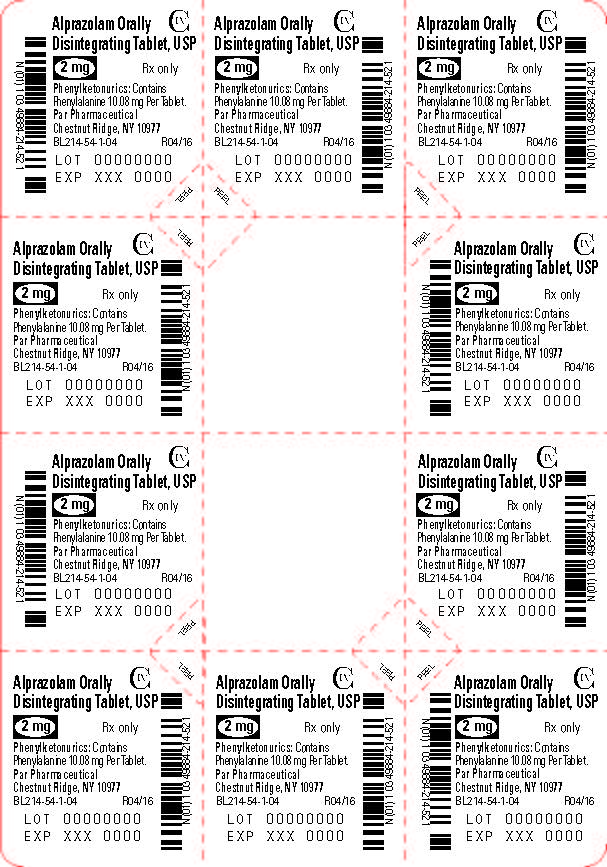
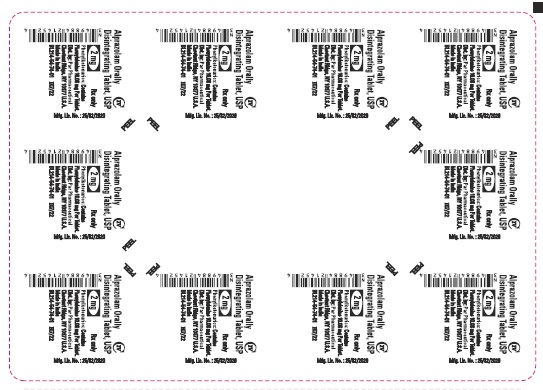
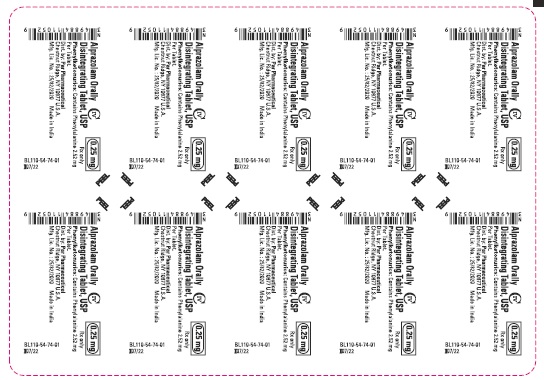
Phenylketonuric patients should be informed that alprazolam orally disintegrating tablets contain phenylalanine (a component of aspartame). Each 0.25 mg, 0.5 mg, 1 mg and 2 mg orally disintegrating tablet contains 2.52 mg, 5.04 mg, 5.04 mg and 10.08 mg of phenylalanine, respectively [see Description (11.1)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
The most commonly reported (greater than or equal to 5% and ~ twice the rate of placebo) adverse reactions with alprazolam treatment are: sedation, impaired coordination, dysarthria, and increased libido.
The data cited in the two tables below are estimates of adverse reactions occurring in patients who participated in clinical trials under the following conditions: relatively short duration (four weeks) placebo-controlled clinical studies with dosages up to 4 mg per day of alprazolam (for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety) and short-term (up to ten weeks) placebo-controlled clinical studies with dosages up to 10 mg per day of alprazolam in patients with panic disorder, with or without agoraphobia.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Comparison of the cited figures, however, can provide the prescriber with some basis for estimating the relative contributions of drug and non-drug factors to the adverse reaction incidence in the population studied. Even this use must be approached cautiously, as a drug may relieve a symptom in one patient but induce it in others. (For example, an anxiolytic drug may relieve dry mouth [a symptom of anxiety] in some subjects but induce dry mouth in others.)
Table 1: Adverse Reactions Reported in Placebo-Controlled Trials of Alprazolam in Generalized Anxiety Disorder (>2% and at a rate greater than placebo) GENERALIZED ANXIETY DISORDER Body System/Adverse Reaction
Treatment-Emergent Symptom Incidencea
ALPRAZOLAM (%)
N=565
PLACEBO (%)
N=505
Central Nervous System
Sedation
41
22
Lightheadedness
21
19
Dizziness
2
1
Akathisia
2
1
Gastrointestinal
Dry Mouth
15
13
Increased Salivation
4
2
Cardiovascular
Hypotension
5
2
Cutaneous
Dermatitis/Allergy
4
3
aEvents reported by 1% or more of alprazolam patients are included.
In addition to the relatively common (i.e., greater than 1%) adverse reactions described in the table above, the following adverse reactions have been reported in association with the use of benzodiazepines: dystonia, irritability, concentration difficulties, anorexia, transient amnesia or memory impairment, loss of coordination, fatigue, seizures, sedation, slurred speech, jaundice, musculoskeletal weakness, pruritus, diplopia, dysarthria, changes in libido, menstrual irregularities, incontinence and urinary retention.
Table 2: Adverse Reactions Reported in Placebo-Controlled Trials of Alprazolam in Panic Disorder (>2 % and greater than placebo) PANIC DISORDER Body System/Adverse Reaction Treatment-Emergent Symptom Incidencea
Central Nervous System
ALPRAZOLAM (%)
N=1388
PLACEBO (%)
N=1231
Sedation
77
43
Fatigue and Tiredness
49
42
Impaired Coordination
40
18
Irritability
33
30
Memory Impairment
33
22
Cognitive Disorder
29
21
Dysarthria
23
6
Decreased Libido
14
8
Confusional State
10
8
Increased Libido
8
4
Change in Libido (Not Specified)
7
6
Disinhibition
3
2
Talkativeness
2
1
Derealization
2
1
Gastrointestinal
Constipation
26
15
Increased Salivation
6
4
Cutaneous
Rash
11
8
Other
Increased Appetite
33
23
Decreased Appetite
28
24
Weight Gain
27
18
Weight Loss
23
17
Micturition Difficulties
12
9
Menstrual Disorders
10
9
Sexual Dysfunction
7
4
Incontinence
2
1
aEvents reported by 1% or more of alprazolam patients are included.
In addition to the relatively common (i.e., greater than 1%) adverse reactions described in the table above, the following adverse reactions have been reported in association with the use of alprazolam: seizures, hallucinations, depersonalization, taste alterations, diplopia, elevated bilirubin, elevated hepatic enzymes, and jaundice.
Panic disorder has been associated with primary and secondary major depressive disorders and increased reports of suicide among untreated patients [see Warnings and Precautions (5.4)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of alprazolam. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Reported events include: liver enzyme elevations, hepatitis, hepatic failure, Stevens-Johnson syndrome, hyperprolactinemia, gynecomastia, and galactorrhea.
-
7 DRUG INTERACTIONS
7.1 Use with Other CNS Depressants
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
If alprazolam orally disintegrating tablets are coadministered with other psychotropic agents or anticonvulsant drugs, carefully consider the pharmacology of the agents to be employed, particularly with compounds which might potentiate the action of benzodiazepines. The benzodiazepines, including alprazolam, produce additive CNS depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistaminics, alcohol and other drugs which themselves produce CNS depression.
7.2 Drugs Effecting Salivary Flow and Stomach pH
Because alprazolam orally disintegrating tablets disintegrate in the presence of saliva, and the formulation requires an acidic environment to dissolve, concomitant drugs or diseases that cause dry mouth or raise stomach pH might slow disintegration or dissolution, resulting in slowed or decreased absorption.
7.3 Use with Imipramine and Desipramine
The steady state plasma concentrations of imipramine and desipramine can increase by approximately 30% and 20%, respectively, when administered concomitantly with alprazolam in doses up to 4 mg per day. The clinical significance of these changes is unknown.
7.4 Drugs that Inhibit Alprazolam Metabolism via Cytochrome P450 3A
The initial step in alprazolam metabolism is hydroxylation catalyzed by cytochrome P450 3A (CYP3A). Drugs which inhibit this metabolic pathway can have a profound effect on the clearance of alprazolam [see Contraindications (4) and Warnings and Precautions (5.8)].
7.5 Drugs Demonstrated to be CYP3A Inhibitors of Possible Clinical Significance on the Basis of Clinical Studies Involving Alprazolam
Use caution during coadministration of Alprazolam and the following drugs:
Fluoxetine — Coadministration of fluoxetine with alprazolam increased the maximum plasma concentration of alprazolam by 46%, decreased clearance by 21%, increased half-life by 17%, and decreased measured psychomotor performance.
Propoxyphene — Coadministration of propoxyphene decreased the maximum plasma concentration of alprazolam by 6%, decreased clearance by 38%, and increased half-life by 58%.
Oral Contraceptives — Coadministration of oral contraceptives increased the maximum plasma concentration of alprazolam by 18%, decreased clearance by 22%, and increased half-life by 29%.
7.6 Drugs and Other Substances Demonstrated to be CYP3A Inhibitors on the Basis of Clinical Studies Involving Benzodiazepines Metabolized Similarly to Alprazolam or on the Basis of In Vitro Studies with Alprazolam or Other Benzodiazepines
Use caution during the coadministration of Alprazolam and the following:
Available data from clinical studies of benzodiazepines other than alprazolam suggest a possible drug interaction between alprazolam and the following: diltiazem, isoniazid, macrolide antibiotics such as erythromycin and clarithromycin, and grapefruit juice. Data from in vitro studies of alprazolam suggest a possible drug interaction between alprazolam and the following: sertraline and paroxetine. However, data from an in vivo drug interaction study involving a single dose of alprazolam 1 mg and steady state doses of sertraline (50 mg to 150 mg per day) did not reveal any clinically significant changes in the pharmacokinetics of alprazolam. Data from in vitro studies of benzodiazepines other than alprazolam suggest a possible drug interaction between benzodiazepines and the following: ergotamine, cyclosporine, amiodarone, nicardipine, and nifedipine [see Warnings and Precautions (5.8)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to psychiatric medication, including Alprazolam orally disintegrating tablets, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late stages in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal [see Warnings and Precautions (5.8) and Clinical Considerations)]. Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear drug association with benzodiazepines and major birth defects (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal adverse reactions
Benzodiazepines cross the placenta and may produce respiratory depression, and sedation in neonates. Monitor neonates exposed to alprazolam during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems. Monitor neonates exposed to alprazolam during pregnancy for signs of withdrawal. Manage these neonates accordingly [see Warnings and Precautions (5.8)].
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco, and other medications, have not confirmed these findings.
8.2 Lactation
Risk Summary
Limited data from published literature reports the presence of alprazolam in human breast milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of alprazolam on lactation are unknown.
Because of the potential for serious adverse reactions, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with Alprazolam orally disintegrating tablets.
8.4 Pediatric Use
Safety and effectiveness of alprazolam in individuals below 18 years of age have not been studied.
8.5 Geriatric Use
The elderly may be more sensitive to the effects of benzodiazepines. They exhibit higher plasma alprazolam concentrations due to reduced clearance of the drug, compared with a younger population receiving the same doses. The smallest effective dose of alprazolam should be used in the elderly to preclude the development of ataxia and oversedation [see Clinical Pharmacology (12) and Dosage and Administration (2)].
Changes in the absorption, distribution, metabolism and excretion of benzodiazepines have been demonstrated in geriatric patients. A mean half-life of alprazolam of 16.3 hours has been observed in healthy elderly subjects (range: 9.0 to 26.9 hours, n=16) compared to 11.0 hours (range: 6.3 to 15.8 hours, n=16) in healthy adult subjects.
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Alprazolam orally disintegrating tablet is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders [see Warnings and Precautions (5.2)].
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
9.3 Dependence
Physical Dependence
Alprazolam orally disintegrating tablets may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use [see Warnings and Precautions (5.3)].
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue alprazolam orally disintegrating tablets or reduce the dosage [see Dosage and Administration (2.3) and Warnings and Precautions (5.3)].
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there may be difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to alprazolam orally disintegrating tablets may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of alprazolam orally disintegrating tablets may develop; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
-
10 OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal [see Warnings and Precautions (5.2)]. Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g., tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of a potentially life-threatening condition (e.g., status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting a poison center (1-800-222-1222), poisoncontrol.org, or medical toxicologist for additional overdosage management recommendations.
-
11 DESCRIPTION
Alprazolam orally disintegrating tablets, USP contain alprazolam, USP which is a triazolo analog of the 1,4 benzodiazepine class of central nervous system-active compounds.
Alprazolam orally disintegrating tablets, USP are an orally administered formulation of alprazolam, USP which rapidly disintegrates on the tongue and does not require water to aid dissolution or swallowing.
The chemical name of alprazolam, USP is 8-Chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-α] [1,4] benzodiazepine. The molecular formula is C17H13CIN4 and the molecular weight is 308.76.
The structural formula is:
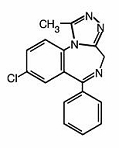
Alprazolam, USP is a white crystalline powder, which is soluble in methanol or ethanol but which has no appreciable solubility in water at physiological pH.
11.1 Alprazolam Orally Disintegrating Tablets, USP
Each orally disintegrating tablet contains either 0.25 mg, 0.5 mg, 1 mg, or 2 mg of alprazolam, USP and the following inactive ingredients: aspartame, crospovidone, basic butylated methacrylate copolymer, magnesium stearate, mannitol, silicon dioxide, sorbitol, talc, xylitol, natural peppermint flavor, artificial vanillin flavor.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The exact mechanism of action of alprazolam is unknown. Benzodiazepines bind to gamma aminobutyric acid (GABA) receptors in the brain and enhance GABA-mediated synaptic inhibition; such actions may be responsible for the efficacy of alprazolam in anxiety disorder and panic disorder.
12.3 Pharmacokinetics
Absorption
Following oral administration, alprazolam is readily absorbed. The peak plasma concentration is reached about 1.5 to 2 hours after administration of alprazolam given with or without water. When taken with water, mean Tmax occurs about 15 minutes earlier than when taken without water with no change in Cmax or AUC. Plasma levels are proportional to the dose given; over the dose range of 0.5 mg to 3 mg, peak levels of 8 to 37 ng/mL are observed. The elimination half-life of alprazolam is approximately 12.5 hours (range 7.9 to 19.2 hours) after administration of alprazolam in healthy adults.
Food decreased the mean Cmax by about 25% and increased the mean Tmax by 2 hours from 2.2 hours to 4.4 hours after the ingestion of a high-fat meal. Food did not affect the extent of absorption (AUC) or the elimination half-life.
Distribution
In vitro, alprazolam is bound (80 percent) to human serum protein. Serum albumin accounts for the majority of the binding.
Metabolism/Elimination
Alprazolam is extensively metabolized in humans, primarily by cytochrome P450 3A4 (CYP3A4), to two major metabolites in the plasma: 4-hydroxyalprazolam and α-hydroxyalprazolam. A benzophenone derived from alprazolam is also found in humans. Their half-lives appear to be similar to that of alprazolam. The plasma concentrations of 4-hydroxyalprazolam and α-hydroxyalprazolam relative to unchanged alprazolam concentration were always less than 4%. The reported relative potencies in benzodiazepine receptor binding experiments and in animal models of induced seizure inhibition are 0.20 and 0.66, respectively, for 4-hydroxyalprazolam and α-hydroxyalprazolam. Such low concentrations and the lesser potencies of 4-hydroxyalprazolam and α-hydroxyalprazolam suggest that they are unlikely to contribute much to the pharmacological effects of alprazolam. The benzophenone metabolite is essentially inactive.
Alprazolam and its metabolites are excreted primarily in the urine.
Special Populations
Changes in the absorption, distribution, metabolism and excretion of benzodiazepines have been reported in a variety of disease states including alcoholism, impaired hepatic function and impaired renal function. Changes have also been demonstrated in geriatric patients. A mean half-life of alprazolam of 16.3 hours has been observed in healthy elderly subjects (range: 9.0 to 26.9 hours, n=16) compared to 11.0 hours (range: 6.3 to 15.8 hours, n=16) in healthy adult subjects. In patients with alcoholic liver disease, the half-life of alprazolam ranged between 5.8 and 65.3 hours (mean: 19.7 hours, n=17) as compared to between 6.3 and 26.9 hours (mean=11.4 hours, n=17) in healthy subjects. In an obese group of subjects, the half-life of alprazolam ranged between 9.9 and 40.4 hours (mean=21.8 hours, n=12) as compared to between 6.3 and 15.8 hours (mean=10.6 hours, n=12) in healthy subjects.
Because of its similarity to other benzodiazepines, it is assumed that alprazolam undergoes transplacental passage and that it is excreted in human milk.
Race — Maximal concentrations (Cmax) and half-life of alprazolam are approximately 15% and 25% higher in Asians compared to Caucasians.
Pediatrics — The pharmacokinetics of alprazolam in pediatric patients have not been studied.
Gender — Gender has no effect on the pharmacokinetics of alprazolam.
Cigarette Smoking — Alprazolam concentrations may be reduced by up to 50% in smokers compared to non-smokers.
Drug-Drug Interactions
Alprazolam is primarily eliminated by metabolism via cytochrome P450 3A (CYP3A). Most of the interactions that have been documented with alprazolam are with drugs that inhibit or induce CYP3A.
Compounds that are potent inhibitors of CYP3A would be expected to increase plasma alprazolam concentrations. Drug products that have been studied in vivo, along with their effect on increasing alprazolam AUC, are as follows: ketoconazole, 3.98 fold; itraconazole, 2.70 fold; nefazodone, 1.98 fold; fluvoxamine, 1.96 fold; and erythromycin, 1.61 fold [see Contraindications (4), Warnings and Precautions (5.8), and Drug Interactions (7)].
CYP3A inducers would be expected to decrease alprazolam concentrations and this has been observed in vivo. The oral clearance of alprazolam (given in a 0.8 mg single dose) was increased from 0.90 ± 0.21 mL/min/kg to 2.13 ± 0.54 mL/min/kg and the elimination t1/2 was shortened (from 17.1 ± 4.9 to 7.7 ± 1.7 h) following administration of 300 mg/day carbamazepine for 10 days [see Drug Interactions (7)]. However, the carbamazepine dose used in this study was fairly low compared to the recommended doses (1000 mg to 1200 mg/day); the effect at usual carbamazepine doses is unknown.
The ability of alprazolam to induce or inhibit human hepatic enzyme systems has not been determined. However, this is not a property of benzodiazepines in general. Further, alprazolam did not affect the prothrombin or plasma warfarin levels in male volunteers administered sodium warfarin orally.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No evidence of carcinogenic potential was observed during 2-year bioassay studies of alprazolam in rats at doses up to 30 mg/kg per day (30 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis) and in mice at doses up to 10 mg/kg per day (5 times the maximum recommended human dose on a mg/m2).
Alprazolam also was not mutagenic in vitro in the DNA Damage/Alkaline Elution Assay or the Ames Assay, and was negative in the rat micronucleus test.
Alprazolam produced no impairment of fertility in rats at doses up to 5 mg/kg per day, which is 5 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
When rats were treated with oral alprazolam doses of 3, 10, and 30 mg/kg per day (3 to 30 times the maximum recommended human dose of 10 mg per day on a mg/m2 basis) for 2 years, a tendency for a dose related increase in the number of cataracts was observed in females, and a tendency for a dose related increase in corneal vascularization was observed in males. These lesions did not appear until after 11 months of treatment.
-
14 CLINICAL STUDIES
14.1 Anxiety Disorders
The efficacy of alprazolam in the treatment of anxiety symptoms was demonstrated in five short-term (4 weeks), randomized, double-blind, placebo-controlled studies. The studies included patients with a diagnosis of anxiety or anxiety with associated depressive symptomatology. Alprazolam doses ranged from 0.5 to 4 mg per day. The mean daily doses ranged from 1.6 to 2.4 mg. Treatment with alprazolam was statistically significantly superior to placebo treatment, as measured by the following psychometric instruments: Hamilton Anxiety Rating Scale, Physician’s Global Impressions, Target Symptoms, Patient’s Global Impressions and Self-Rating Symptom Scale.
14.2 Panic Disorder
The efficacy of alprazolam in the treatment of panic disorder was demonstrated in three short-term (up to 10 weeks), randomized, double-blind, placebo-controlled studies. Patients in the studies had diagnoses corresponding closely to DSM-III-R criteria for panic disorder (with or without agoraphobia).
The average dose of alprazolam was 5 mg to 6 mg per day in two of the studies, and the doses of alprazolam were fixed at 2 mg and 6 mg per day in the third study. In all three studies, alprazolam was superior to placebo on a variable defined as "the number of patients with zero panic attacks" (range, 37 to 83% met this criterion), as well as on a global improvement score. In two of the three studies, alprazolam was superior to placebo on a variable defined as "change from baseline on the number of panic attacks per week" (range, 3.3 to 5.2), and also on a phobia rating scale. A subgroup of patients who were improved on alprazolam during short-term treatment in one of these trials was continued on an open basis up to 8 months, without apparent loss of benefit.
-
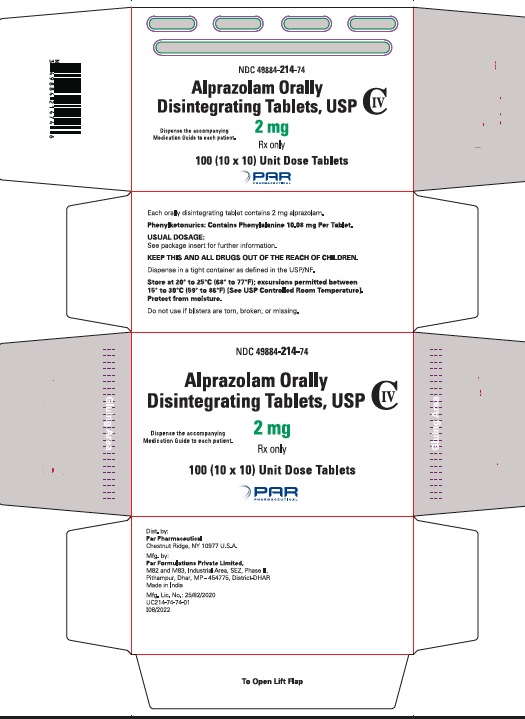
16 HOW SUPPLIED/STORAGE AND HANDLING
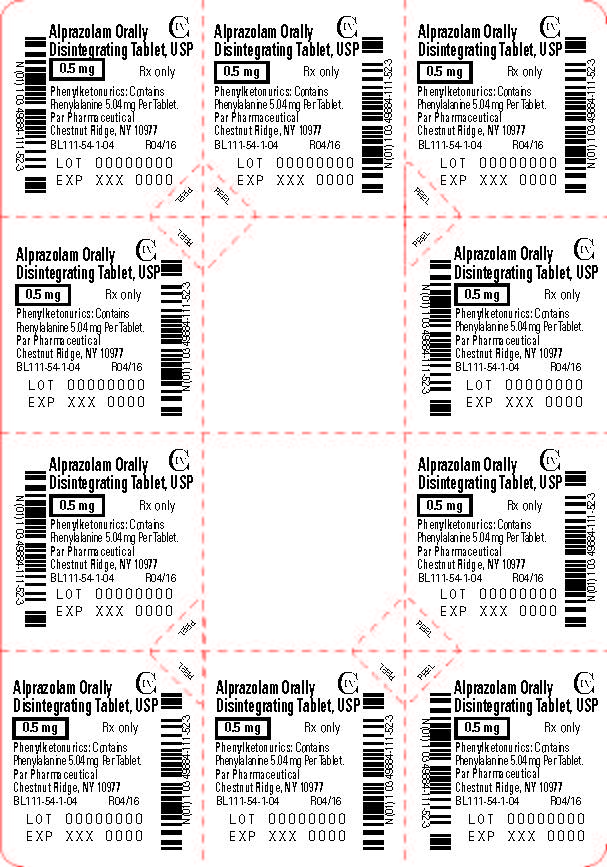
Alprazolam orally disintegrating tablets, USP 0.25 mg are white to off-white, round, scored, flat face, beveled edge tablets debossed ‘110’ on the scored side and plain on the other. They are supplied as follows:
Blisters of 10 x 10 NDC 49884-110-74
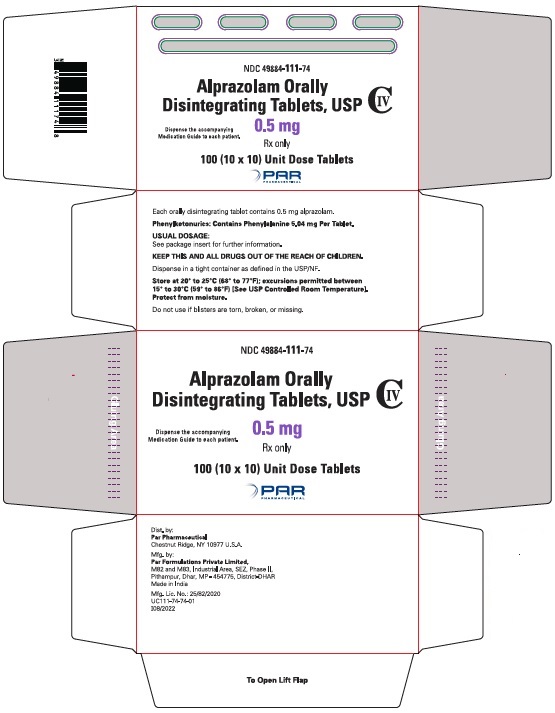
Alprazolam orally disintegrating tablets, USP 0.5 mg are white to off-white, round, scored, flat face, beveled edge tablets debossed ‘111’ on the scored side and plain on the other. They are supplied as follows:
Blisters of 10 x 10 NDC 49884-111-74
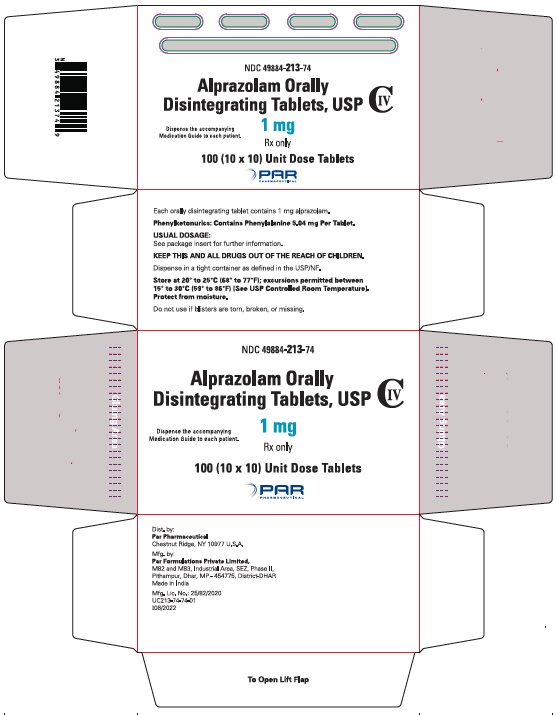
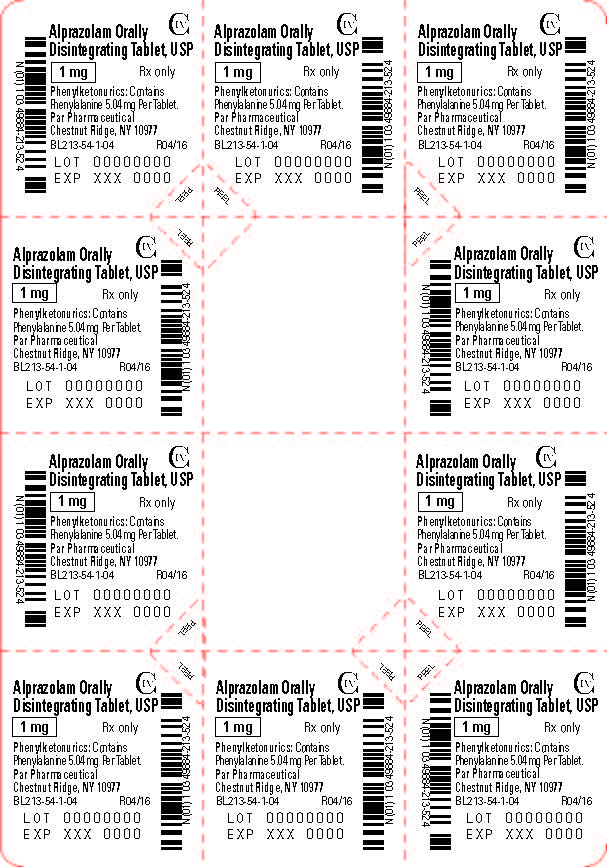
Alprazolam orally disintegrating tablets, USP 1 mg are white to off-white, round, scored, flat face, beveled edge tablets debossed ‘213’ on the scored side and plain on the other. They are supplied as follows:
Blisters of 10 x 10 NDC 49884-213-74
Alprazolam orally disintegrating tablets, USP 2 mg are white to off-white, round, scored, flat face, beveled edge tablets debossed ‘214’ on the scored side and plain on the other. They are supplied as follows:
Blisters of 10 x 10 NDC 49884-214-74
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86ºF) [See USP Controlled Room Temperature]. Protect from moisture.
Dispense in tight, light-resistant containers as defined in the USP.
Keep container tightly closed.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Inform patients and caregivers that potentially fatal additive effects may occur if alprazolam is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)].
Abuse, Misuse, and Addiction
Inform patients that the use of Alprazolam orally disintegrating tablets, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug [see Warnings and Precautions (5.2) and Drug Abuse and Dependence (9.2)].
Withdrawal Reactions
Inform patients that the continued use of Alprazolam orally disintegrating tablets may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of Alprazolam orally disintegrating tablets may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of alprazolam orally disintegrating tablets may require a slow taper [see Warnings and Precautions (5.3) and Drug Abuse and Dependence (9.3)].
Use/Handling Alprazolam Orally Disintegrating Tablets
To assure safe and effective use of benzodiazepines, all patients prescribed alprazolam orally disintegrating tablets should be provided with the following guidance.
- Do not remove Alprazolam orally disintegrating tablets from the blister until just prior to dosing. With dry hands, open the blister, remove the tablet, and immediately place on the tongue to dissolve and be swallowed with the saliva. The tablet may also be taken with water.
- Store at room temperature in a dry place. Protect from moisture.
- Inform your physician about any alcohol consumption and medicine you are taking now, including medication you may buy without a prescription. Alcohol should generally not be used during treatment with benzodiazepines.
- Alprazolam orally disintegrating tablets are not recommended for use in pregnancy. Therefore, inform your physician if you are pregnant, if you are planning to have a child, or if you become pregnant while you are taking this medication.
- Inform your physician if you are nursing.
- Until you experience how this medication affects you, do not drive a car or operate potentially dangerous machinery, etc.
- Do not increase the dose even if you think the medication “does not work anymore” without consulting your physician. Benzodiazepines, even after relatively short-term use at the doses recommended, may produce emotional and/or physical dependence.
- Do not stop taking this medication abruptly or decrease the dose without consulting your physician, since withdrawal symptoms can occur even after relatively short-term use at the doses recommended. You should follow a gradual dosage tapering schedule.
- Pregnancy: Advise pregnant females that use of Alprazolam orally disintegrating tablets late in pregnancy can result in sedation, (respiratory depression, withdrawal symptoms, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns [see Warnings and Precautions (5.8), Use in Specific Populations (8.1)]. Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Alprazolam orally disintegrating tablets during pregnancy [see Use in Specific Populations (8.1)].
10. Phenylketonurics: Phenylketonuric patients should be informed that alprazolam orally disintegrating tablets contain phenylalanine (a component of aspartame). Each 0.25 mg, 0.5 mg, 1 mg and 2 mg orally disintegrating tablet contains 2.52 mg, 5.04 mg, 5.04 mg and 10.08 mg of phenylalanine, respectively [see Description (11.1)].
11. Lactation: Advise patients that breastfeeding is not recommended during treatment with Alprazolam orally disintegrating tablets [see Use in Specific Populations (8.2)].
Dispense with Medication Guide available at: www.parpharm.com.
Distributed by:
PAR PHARMACEUTICAL
Chestnut Ridge, NY 10977
-
MEDICATION GUIDE
Alprazolam (al pra′ zoe lam)
Orally Disintegrating Tablet (ODT), C-IV
-
What is the most important information I should know about alprazolam ODT?
Alprazolam ODT is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system (CNS) depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
Do not drive or operate heavy machinery until you know how taking alprazolam ODT with opioids affects you.
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including alprazolam ODT which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including Alprazolam ODT. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take Alprazolam ODT exactly as prescribed by your healthcare provider.
- Take Alprazolam ODT exactly as your healthcare provider prescribed.
- Do not share your Alprazolam ODT with other people.
- Keep Alprazolam ODT in a safe place and away from children.
- Physical dependence and withdrawal reactions. Alprazolam ODT can cause physical dependence and withdrawal reactions, especially if you continue to take Alprazolam ODT for several days to several weeks.
- Do not suddenly stop taking Alprazolam ODT. Stopping Alprazolam ODT suddenly can cause serious and life-threatening side effects, including, unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more Alprazolam ODT than prescribed or take Alprazolam ODT for longer than prescribed.
What is alprazolam ODT?
- Alprazolam ODT is a prescription medicines used to treat:
- generalized anxiety disorder
- panic disorder with or without fear of places and situations that might cause panic, helplessness, or embarrassment (agoraphobia)
- Alprazolam ODT is a federal controlled substance (C-IV) because it contains alprazolam that can be abused or lead to dependence. Keep alprazolam ODT in a safe place to prevent misuse and abuse. Selling or giving away alprazolam ODT may harm others, and is against the law. Tell your healthcare provider if you have abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if alprazolam ODT is safe and effective in children.
- It is not known if alprazolam ODT is safe and effective when to treat generalized anxiety disorder for longer than 4 months.
- It is not known if alprazolam ODT is safe and effective when used to treat panic disorder for longer than 10 weeks.
Do not take alprazolam ODT if you:
- take an antifungal medicines including ketoconazole and itraconazole
Before you take alprazolam ODT, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have liver or kidney problems
- have lung disease or breathing problems
- are pregnant or plan to become pregnant. You and your healthcare provider should decide if you should take alprazolam ODT while you are pregnant.
- Taking alprazolam ODT late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with alprazolam ODT.
- There is a pregnancy registry for women who take alprazolam ODT during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with alprazolam ODT, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting https://womensmentalhealth.org/research/pregnancyregistry/.
- are breastfeeding or plan to breastfeed. Alprazolam passes into your breast milk
- Talk to your healthcare provider about the best way to feed your baby if you take alprazolam ODT.
- Breastfeeding is not recommended during treatment with alprazolam ODT.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking alprazolam ODT with certain other medicines can cause side effects or affect how well alprazolam ODT or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
How should I take alprazolam ODT?
- Take alprazolam ODT exactly as your healthcare provider tells you to take it. Your healthcare provider will tell you how much alprazolam ODT to take and when to take it.
- Do not remove alprazolam ODT from the blister until you are ready to take it. With dry hands, remove the alprazolam ODT, and immediately place on your tongue to dissolve and be swallowed with your saliva. The tablet may also be taken with water.
- If you take too much alprazolam ODT, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of alprazolam ODT?
Alprazolam ODT may cause serious side effects, including:
- See “What is the most important information I should know about alprazolam ODT?”
- Seizures. Stopping alprazolam ODT can cause seizures and seizures that will not stop (status epilepticus).
- Mania. Alprazolam ODT may cause an increase in activity and talking (hypomania and mania) in people who have depression.
-
Alprazolam ODT can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Alprazolam ODT affects you.
- Do not drink alcohol or take other medicines that may make you sleepy or dizzy while taking Alprazolam ODT without first talking to your healthcare provider. When taken with alcohol or other medicines that cause sleepiness or dizziness, Alprazolam ODT may make your sleepiness or dizziness much worse.
The most common side effects of alprazolam ODT include:
- sedation
- problems with coordination
- speech problems (dysarthria)
- increased libido
- low blood pressure (hypotension)
These are not all the possible side effects of alprazolam ODT. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store alprazolam ODT?
- Store alprazolam ODT at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep alprazolam ODT in a tightly closed container and keep dry.
- Keep alprazolam ODT and all medicines out of the reach of children.
General information about the safe and effective use of alprazolam ODT.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use alprazolam ODT for a condition for which it was not prescribed. Do not give alprazolam ODT to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about alprazolam ODT that is written for health professionals. For more information call 1-800-828-9393.
What are the ingredients in alprazolam ODT?
Active ingredients: alprazolam
Inactive ingredients: aspartame, crospovidone, basic butylated methacrylate copolymer, magnesium stearate, mannitol, silicon dioxide, sorbitol, talc, xylitol, natural peppermint flavor, artificial vanillin flavor.
Distributed by:
PAR PHARMACEUTICAL
Chestnut Ridge, NY 10977
This Medication Guide has been approved by the U.S. Food and Drug Administration
-
What is the most important information I should know about alprazolam ODT?
- PRINCIPAL DISPLAY PANEL 0.25 MG TABS CARTON
- PRINCIPAL DISPLAY PANEL 0.25 MG TABS FOIL SEAL
- PRINCIPAL DISPLAY PANEL 0.5 MG TABS CARTON
- PRINCIPAL DISPLAY PANEL 0.5 MG TABS FOILSEAL
- PRINCIPAL DISPLAY PANEL 1 MG TABS CARTON
- PRINCIPAL DISPLAY PANEL 1 MG TABS FOILSEAL
- PRINCIPAL DISPLAY PANEL 2 MG TABS CARTON
- PRINCIPAL DISPLAY PANEL 2 MG TABS FOILSEAL
-
INGREDIENTS AND APPEARANCE
ALPRAZOLAM
alprazolam tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-110 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPRAZOLAM (UNII: YU55MQ3IZY) (ALPRAZOLAM - UNII:YU55MQ3IZY) ALPRAZOLAM 0.25 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) TALC (UNII: 7SEV7J4R1U) XYLITOL (UNII: VCQ006KQ1E) PEPPERMINT (UNII: V95R5KMY2B) VANILLIN (UNII: CHI530446X) Product Characteristics Color white (110) Score 2 pieces Shape ROUND (110) Size 8mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-110-74 10 in 1 CARTON 01/09/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078088 01/09/2009 ALPRAZOLAM
alprazolam tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-111 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPRAZOLAM (UNII: YU55MQ3IZY) (ALPRAZOLAM - UNII:YU55MQ3IZY) ALPRAZOLAM 0.5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) TALC (UNII: 7SEV7J4R1U) XYLITOL (UNII: VCQ006KQ1E) PEPPERMINT (UNII: V95R5KMY2B) VANILLIN (UNII: CHI530446X) Product Characteristics Color white (111) Score 2 pieces Shape ROUND (111) Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-111-74 10 in 1 CARTON 01/09/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078088 01/09/2009 ALPRAZOLAM
alprazolam tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-213 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPRAZOLAM (UNII: YU55MQ3IZY) (ALPRAZOLAM - UNII:YU55MQ3IZY) ALPRAZOLAM 1 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) TALC (UNII: 7SEV7J4R1U) XYLITOL (UNII: VCQ006KQ1E) PEPPERMINT (UNII: V95R5KMY2B) VANILLIN (UNII: CHI530446X) Product Characteristics Color white (213) Score 2 pieces Shape ROUND (213) Size 10mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-213-74 10 in 1 CARTON 01/09/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078088 01/09/2009 ALPRAZOLAM
alprazolam tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:49884-214 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALPRAZOLAM (UNII: YU55MQ3IZY) (ALPRAZOLAM - UNII:YU55MQ3IZY) ALPRAZOLAM 2 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SORBITOL (UNII: 506T60A25R) TALC (UNII: 7SEV7J4R1U) XYLITOL (UNII: VCQ006KQ1E) PEPPERMINT (UNII: V95R5KMY2B) VANILLIN (UNII: CHI530446X) Product Characteristics Color white (214) Score 2 pieces Shape ROUND (214) Size 12mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49884-214-74 10 in 1 CARTON 01/09/2009 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078088 01/09/2009 Labeler - Par Pharmaceutical, Inc. (092733690)