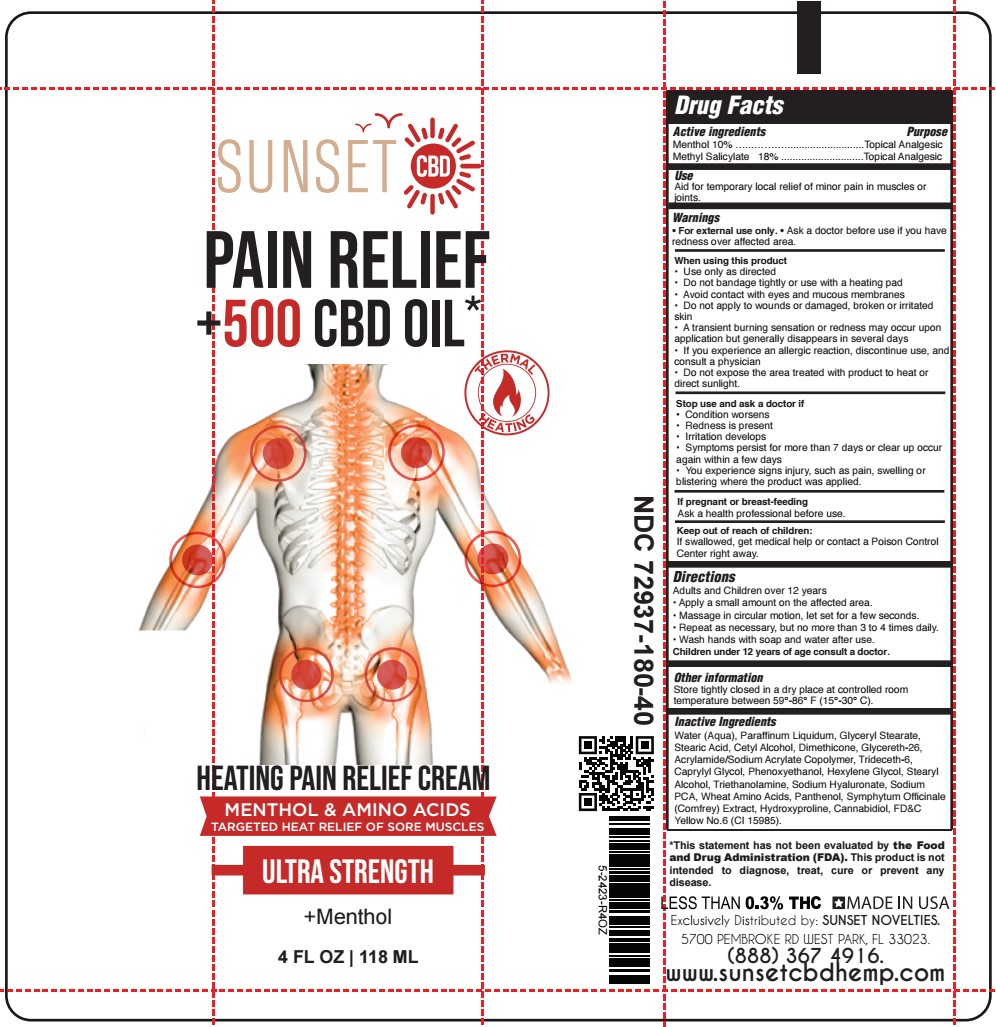
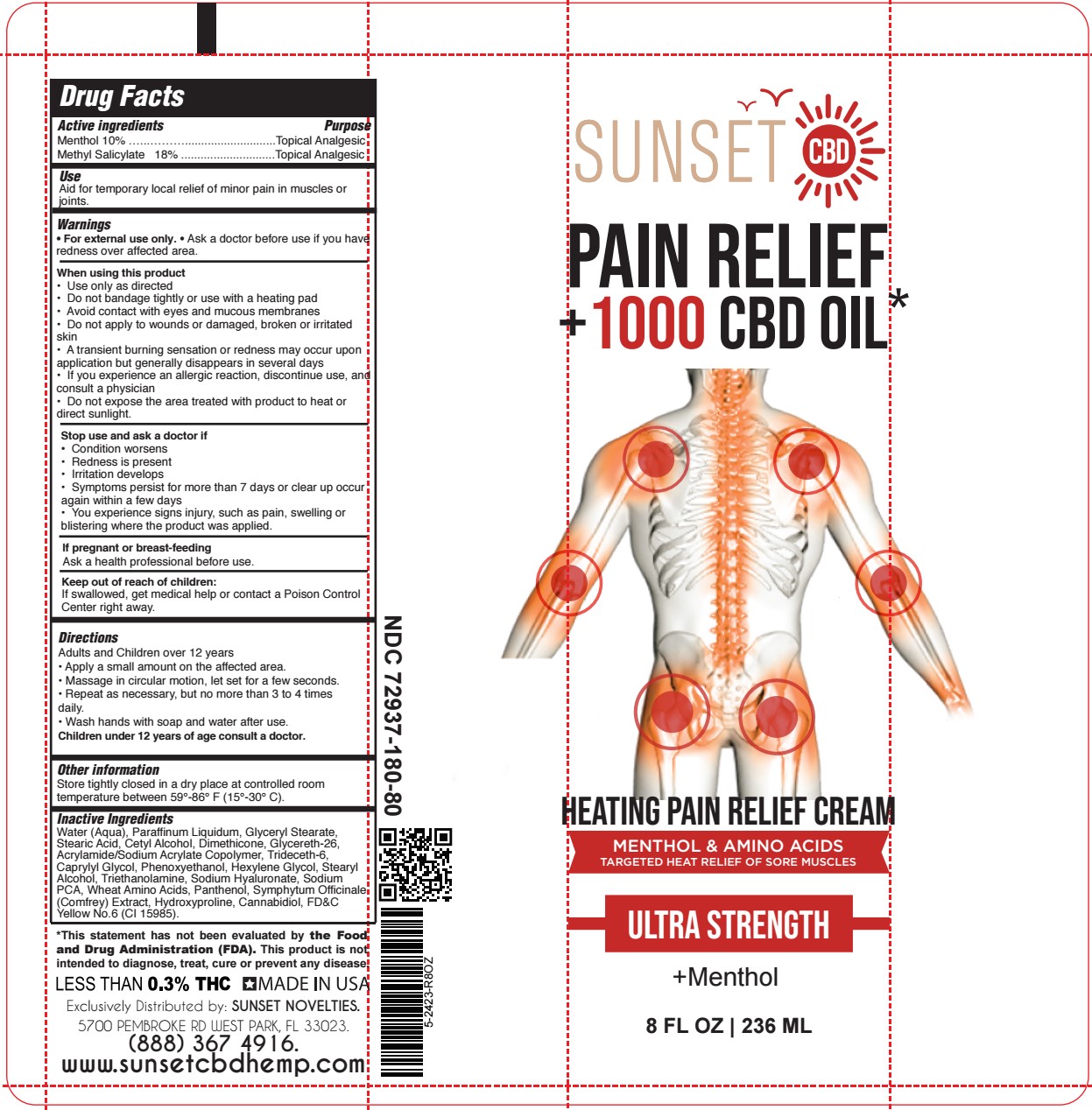
Label: HEATING PAIN RELIEF- methyl salicylate, menthol cream
- NDC Code(s): 72937-180-40, 72937-180-80
- Packager: SUNSET NOVELTIES, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES:
- WARNINGS
-
WHEN USING
Use only as directed
Do not bandage tightly or use with a heating pad
Avoid contact with eyes and mucous membranes
Do not apply to wounds or damaged, broken or irritated skin
If you experience an allergic reaction, discontinue use and consult a doctor.Do not expose the area treated with product to heat or direct sunlight.
- STOP USE AND ASK A DOCTOR IF:
- IF PREGNANT OR BREAST – FEEDING:
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS:
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive Ingredients
Water (Aqua), Paraffinum Liquidum, Glyceryl Stearate, Stearic Acid, Cetyl Alcohol, Dimethicone, Glycereth-26, Acrylamide/Sodium Acrylate Copolymer, Trideceth-6, Caprylyl Glycol, Phenoxyethanol, Hexylene Glycol, Stearyl Alcohol, Triethanolamine, Sodium Hyaluronate, Sodium PCA, Wheat Amino Acids, Panthenol, Symphytum Officinale (Comfrey) Extract, Hydroxyproline, Cannabidiol, FD&C Yellow No.6 (CI 15985).
- SUNSET - HEATING PAIN RELIEF CREAM 4 oz TUBE
- SUNSET - HEATING PAIN RELIEF CREAM 8 oz TUBE
-
INGREDIENTS AND APPEARANCE
HEATING PAIN RELIEF
methyl salicylate, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72937-180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 9.8 g in 100 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 17.6 g in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERETH-26 (UNII: NNE56F2N14) TRIDECETH-6 (UNII: 3T5PCR2H0C) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) COMFREY LEAF (UNII: DG4F8T839X) CANNABIDIOL (UNII: 19GBJ60SN5) WATER (UNII: 059QF0KO0R) HYALURONATE SODIUM (UNII: YSE9PPT4TH) AMINO ACIDS, WHEAT (UNII: 0370GZL32F) PANTHENOL (UNII: WV9CM0O67Z) HYDROXYPROLINE (UNII: RMB44WO89X) HEXYLENE GLYCOL (UNII: KEH0A3F75J) STEARIC ACID (UNII: 4ELV7Z65AP) DIMETHICONE 1000 (UNII: MCU2324216) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) TROLAMINE (UNII: 9O3K93S3TK) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) MINERAL OIL (UNII: T5L8T28FGP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange (Light Orange) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72937-180-40 118 mL in 1 TUBE; Type 0: Not a Combination Product 06/26/2023 2 NDC:72937-180-80 236 mL in 1 TUBE; Type 0: Not a Combination Product 06/26/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/25/2023 Labeler - SUNSET NOVELTIES, INC (067218145)