Label: KYBELLA- deoxycholic acid injection, solution
- NDC Code(s): 61168-101-01, 61168-101-03, 61168-101-04, 61168-101-91, view more
- Packager: Kythera Biopharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use KYBELLA safely and effectively. See full prescribing information for KYBELLA. KYBELLA® (deoxycholic acid) injection, for ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
KYBELLA® (deoxycholic acid) injection is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. Limitations of use - The ...
-
2
DOSAGE AND ADMINISTRATION
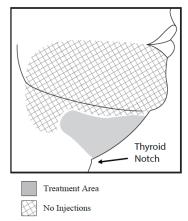
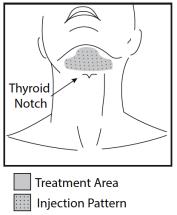
2.1 - Dosage - KYBELLA injection is injected into subcutaneous fat tissue in the submental area using an area-adjusted dose of 2 mg/cm2. A single treatment consists of up to a maximum ...
-
3
DOSAGE FORMS AND STRENGTHS
Injection: 10 mg/mL. KYBELLA (deoxycholic acid) injection is a clear, colorless, sterile solution supplied in 2 mL vials intended for single patient use. Each milliliter of the solution contains ...
-
4
CONTRAINDICATIONS
KYBELLA injection is contraindicated in the presence of infection at the injection sites.
-
5
WARNINGS AND PRECAUTIONS
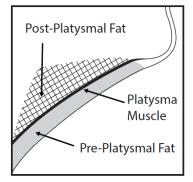
5.1 - Marginal mandibular nerve injury - Cases of marginal mandibular nerve injury, manifested as an asymmetric smile or facial muscle weakness (paresis), were reported during clinical ...
-
6
ADVERSE REACTIONS
6.1 - Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Risk Summary - There are no adequate and well-controlled studies of KYBELLA injection in pregnant women to inform the drug-associated risk. In animal reproduction ...
-
11
DESCRIPTION
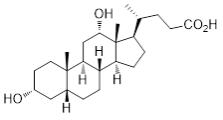
KYBELLA (deoxycholic acid) injection, 10 mg/mL is a clear colorless, sterile solution for subcutaneous use. It contains a cytolytic agent, deoxycholic acid, as the active ingredient. The ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - KYBELLA injection is a cytolytic drug, which when injected into tissue physically destroys the cell membrane causing lysis ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate the carcinogenic potential of KYBELLA ...
-
14
CLINICAL STUDIES
Two randomized, multi-center, double-blind, placebo-controlled trials of identical design were conducted to evaluate KYBELLA injection for use in improvement in the appearance of convexity or ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
KYBELLA (deoxycholic acid) injection, 10 mg/mL is a clear, colorless, sterile solution supplied in 2 mL, single patient use vials in the following dispensing pack: 4 vials, NDC 61168-101-04 - Store ...
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Advise patients to contact their healthcare providers if patients begin to develop signs of marginal mandibular ...
-
PATIENT PACKAGE INSERTPatient Information - KYBELLA® (kye be lah) (deoxycholic acid) injection - What is KYBELLA? KYBELLA is a prescription medicine used in adults to improve the appearance and profile of ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel - NDC: 61168-101-04 - kybella® (deoxycholic acid) injection 10 mg/mL - 20 mg/2 mL - (10 mg/mL) for subcutaneous use only - Single use vials. Discard unused portion. Four ...
-
INGREDIENTS AND APPEARANCEProduct Information