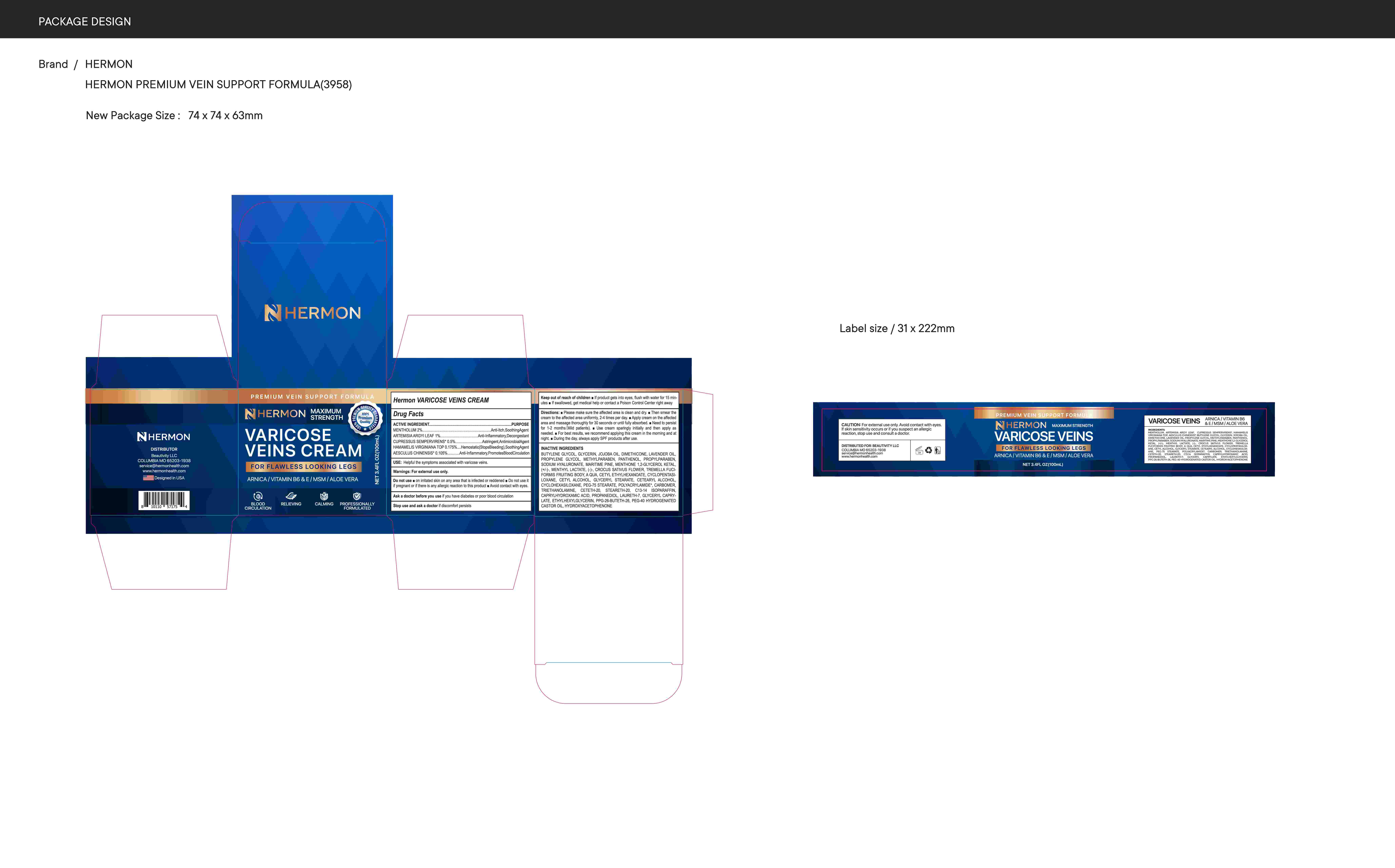
Label: HERMON VARICOSE VEINS CREAM- varicose veins cream cream
- NDC Code(s): 82739-012-01
- Packager: Shenzhen Situya Trading Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- Stop Use
- Keep Oot Of Reach Of Children
- Ask Doctor
-
Directions
Please make sure the affected area is clean and dry.
Then smear the cream to the affected area uniformly, 2-4 times per day.
Apply cream on the affected area and massage thoroughly for 30 seconds or until fully absorbed.
Need to persist for 1-2 months(Mild patients).
Use cream sparingly initially and then apply as needed.
For best results, we recommend applying this cream in the morning and at night.
During the day, always apply SPF products after use.
-
Inactive ingredients
BUTYLENE GLYCOL, GLYCERIN, JOJOBA OIL, DIMETHICONE, LAVENDER OIL, PROPYLENE GLYCOL, METHYLPARABEN, PANTHENOL, PROPYLPARABEN, SODIUM HYALURONATE, MARITIME PINE, MENTHONE 1,2-GLYCEROL KETAL, (+/-)-, MENTHYL LACTATE, (-)-, CROCUS SATIVUS FLOWER, TREMELLA FUCIFORMIS FRUITING BODY, A QUA, CETYL ETHYLHEXANOATE, CYCLOPENTASILOXANE, CETYL ALCOHOL, GLYCERYL STEARATE, CETEARYL ALCOHOL, CYCLOHEXASILOXANE, PEG-75 STEARATE, POLYACRYLAMIDE*, CARBOMER, TRIETHANOLAMINE, CETETH-20, STEARETH-20, C13-14 ISOPARAFFIN, CAPRYLHYDROXAMIC ACID, PROPANEDIOL, LAURETH-7, GLYCERYL CAPRYLATE, ETHYLHEXYLGLYCERIN, PPG-26-BUTETH-26, PEG-40 HYDROGENATED CASTOR OIL, HYDROXYACETOPHENONE
- Questions
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HERMON VARICOSE VEINS CREAM
varicose veins cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82739-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CUPRESSUS SEMPERVIRENS LEAF OIL (UNII: M7QUY89S4O) (CUPRESSUS SEMPERVIRENS LEAF OIL - UNII:M7QUY89S4O) CUPRESSUS SEMPERVIRENS LEAF OIL 0.5 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 g ARTEMISIA ARGYI LEAF (UNII: 2JYC99Q0WZ) (ARTEMISIA ARGYI LEAF - UNII:2JYC99Q0WZ) ARTEMISIA ARGYI LEAF 1 g in 100 g HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) (HAMAMELIS VIRGINIANA TOP - UNII:UDA30A2JJY) HAMAMELIS VIRGINIANA TOP 0.175 g in 100 g AESCULUS CHINENSIS WHOLE (UNII: PGU072XJ6U) (AESCULUS CHINENSIS WHOLE - UNII:PGU072XJ6U) AESCULUS CHINENSIS WHOLE 0.105 g in 100 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 6 (UNII: XHK3U310BA) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) MARITIME PINE (UNII: 50JZ5Z98QY) CETYL ALCOHOL (UNII: 936JST6JCN) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PEG-75 STEARATE (UNII: OT38R0N74H) CETETH-20 (UNII: I835H2IHHX) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) PROPANEDIOL (UNII: 5965N8W85T) PPG-26-BUTETH-26 (UNII: 2II1K6TZ4P) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) JOJOBA OIL (UNII: 724GKU717M) LAVENDER OIL (UNII: ZBP1YXW0H8) PANTHENOL (UNII: WV9CM0O67Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TROLAMINE (UNII: 9O3K93S3TK) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) LAURETH-7 (UNII: Z95S6G8201) GLYCERYL CAPRYLATE (UNII: TM2TZD4G4A) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) MENTHONE 1,2-GLYCEROL KETAL, (+/-)- (UNII: 7QQ1EE6RCP) CROCUS SATIVUS FLOWER (UNII: 00IF91KFKQ) TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) WATER (UNII: 059QF0KO0R) POLYACRYLAMIDE (CROSSLINKED; 0.01-0.2 MOLE PERCENT BISACRYLAMIDE) (UNII: RHA9LWJ494) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) STEARETH-20 (UNII: L0Q8IK9E08) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82739-012-01 100 g in 1 CANISTER; Type 0: Not a Combination Product 06/07/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/07/2023 Labeler - Shenzhen Situya Trading Co., Ltd. (706154255) Establishment Name Address ID/FEI Business Operations Shenzhen Situya Trading Co., Ltd. 706154255 manufacture(82739-012) , label(82739-012)