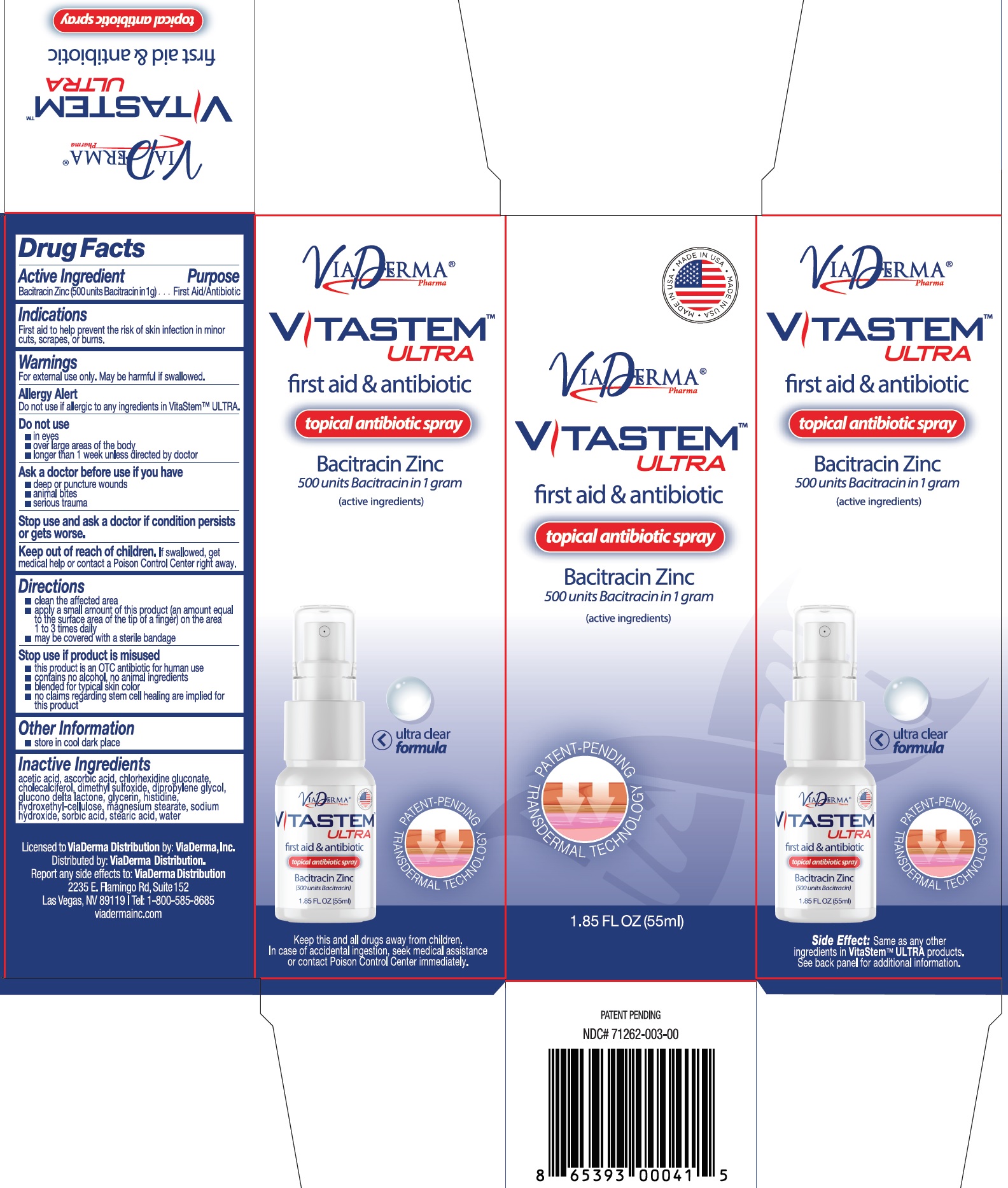
Label: VITASTEM ULTRA- bacitracin zinc ointment
- NDC Code(s): 71262-007-15, 71262-007-55
- Packager: ViaDerma Distribution, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
-
Active Ingredient
Bacitracin Zinc (500 units Bacitracin in 1g) Purpose - First Aid/Antibiotic
-
UsesFirst aid to help prevent the risk of skin infection in minor cuts, scrapes, or burns.
-
WarningsFor external use only. May be harmful if swallowed. Allergy Alert - Do not use if allergic to any ingredients in VitaStem™ ULTRA. Do not use - in eyes - over large areas of the body - longer than 1 ...
-
Directionsclean the affected area - apply a small amount of this product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily - may be covered with a sterile ...
-
Other Informationstore in a cool dry place
-
Inactive Ingredientsacetic acid, ascorbic acid, chlorhexidine gluconate, cholecalciferol, dimethyl sulfoxide, dipropylene glycol, glucono delta lactone, glycerin, histidine, hydroxethyl cellulose, magnesium stearate ...
-
Package Labeling:71262-007-15
 ...
... -
Package Labeling:71262-007-55
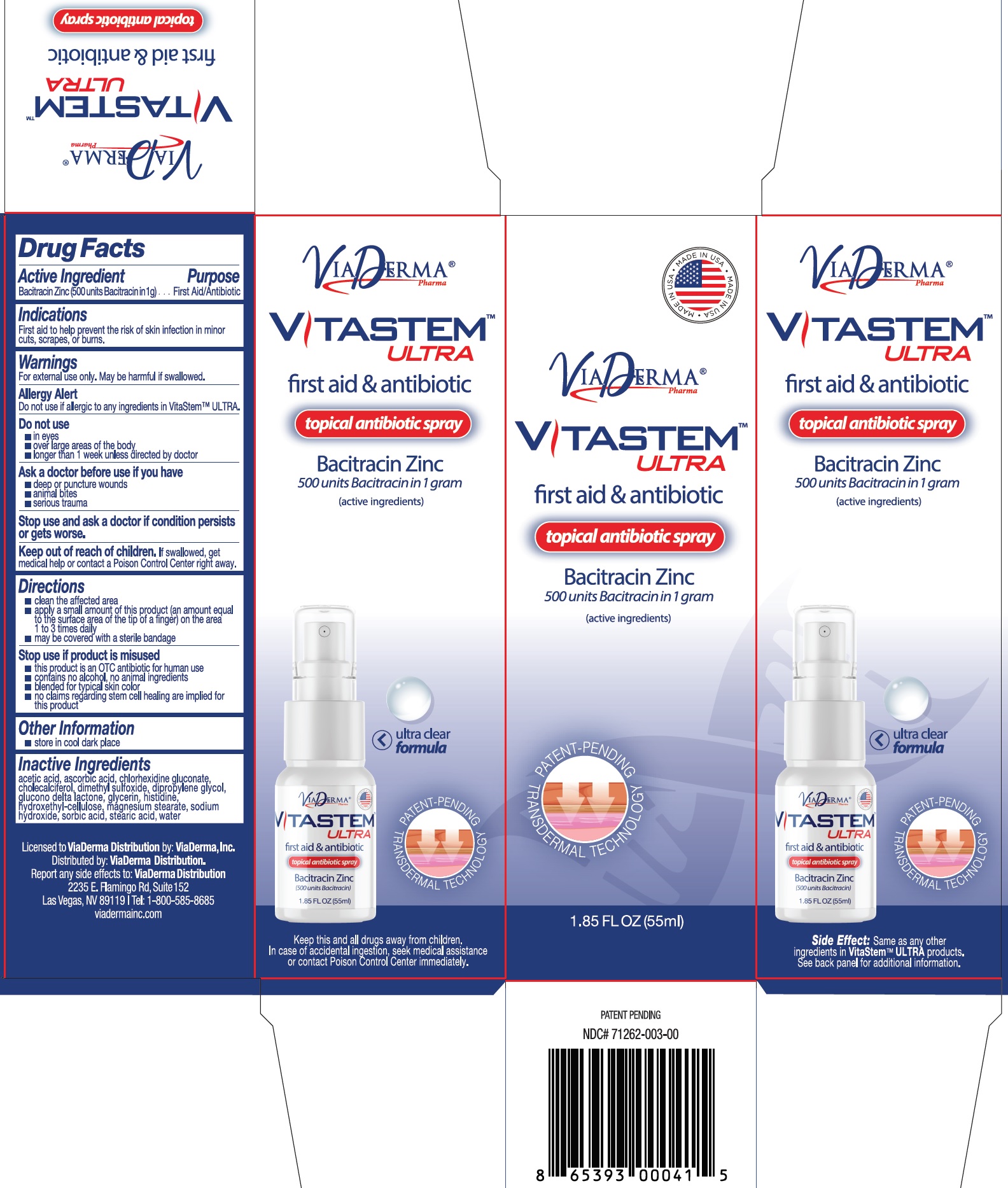 ...
... -
INGREDIENTS AND APPEARANCEProduct Information