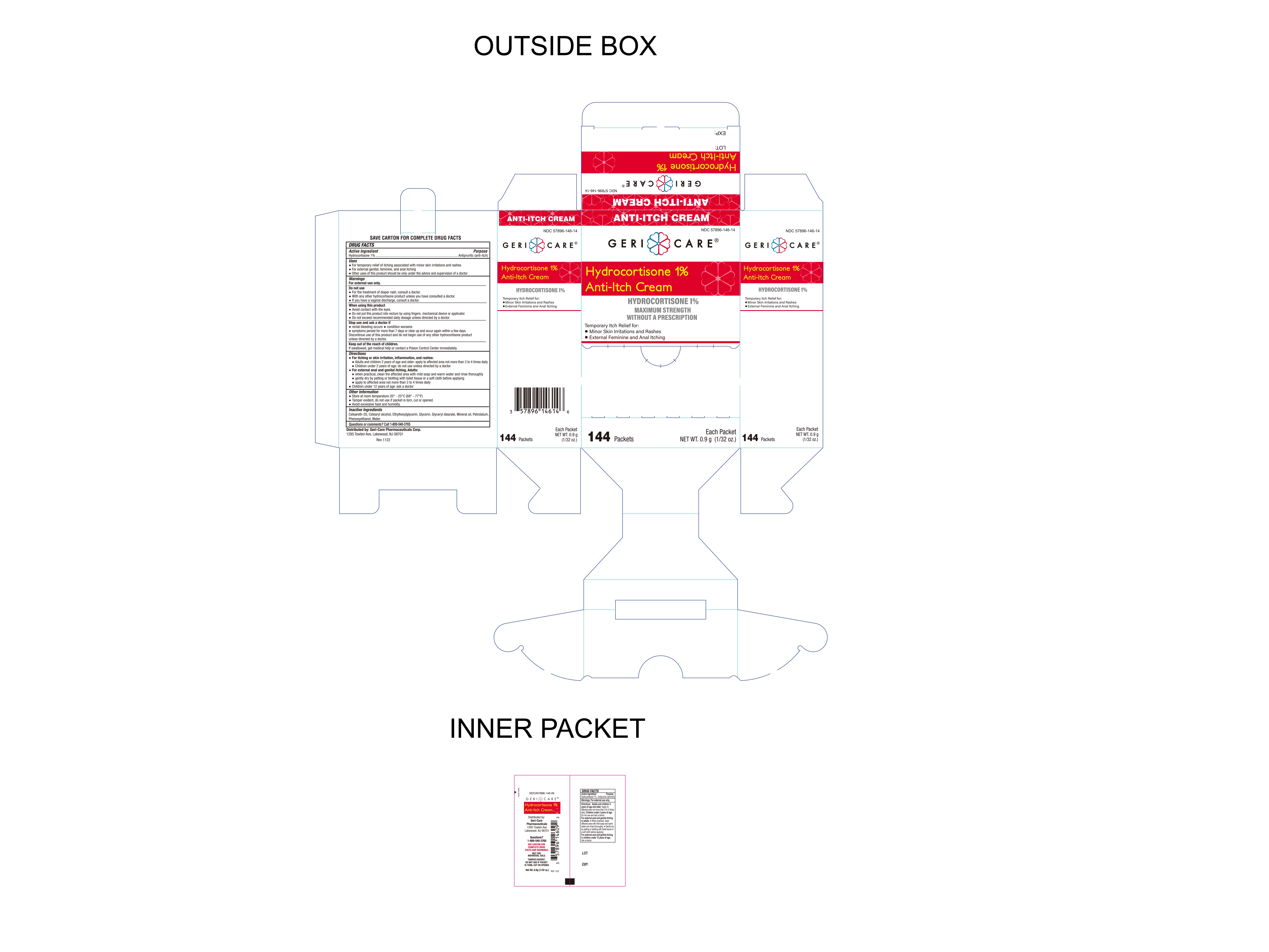
Label: HYDROCORTISONE 1%- hydrocortisone cream
- NDC Code(s): 57896-146-14
- Packager: Geri-Care Pharmaceuticals, Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active ingredient
- Purpose
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Do Not Use
- Warnings
- When using this product
- Stop using this product and ask a doctor if
-
Directions
For Itching or skin irritation, inflammation, and rashes:
Adults and children 2 years of age and older: apply to the affected area not more than 3 to 4 times daily.
Children under 2 years of age: do not use and ask a doctor.
For External and anal itching in adults:
When practical, clean affected area with mild soap and warm water and rinse thoroughly
Gently dry by patting or blotting with toilet tissue or a soft cloth before applying
apply to affected area not more than 3 to 4 times daily.
For children under 12 years of age: Ask a doctor
- Inactive ingredients
- Other information
- Questions
- Distributed By
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE 1%
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57896-146 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength PHENOXYETHANOL (UNII: HIE492ZZ3T) MINERAL OIL (UNII: T5L8T28FGP) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57896-146-14 144 in 1 CARTON 05/31/2023 1 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/31/2023 Labeler - Geri-Care Pharmaceuticals, Corp (611196254) Registrant - Trifecta Pharmaceuticals USA (079424163)