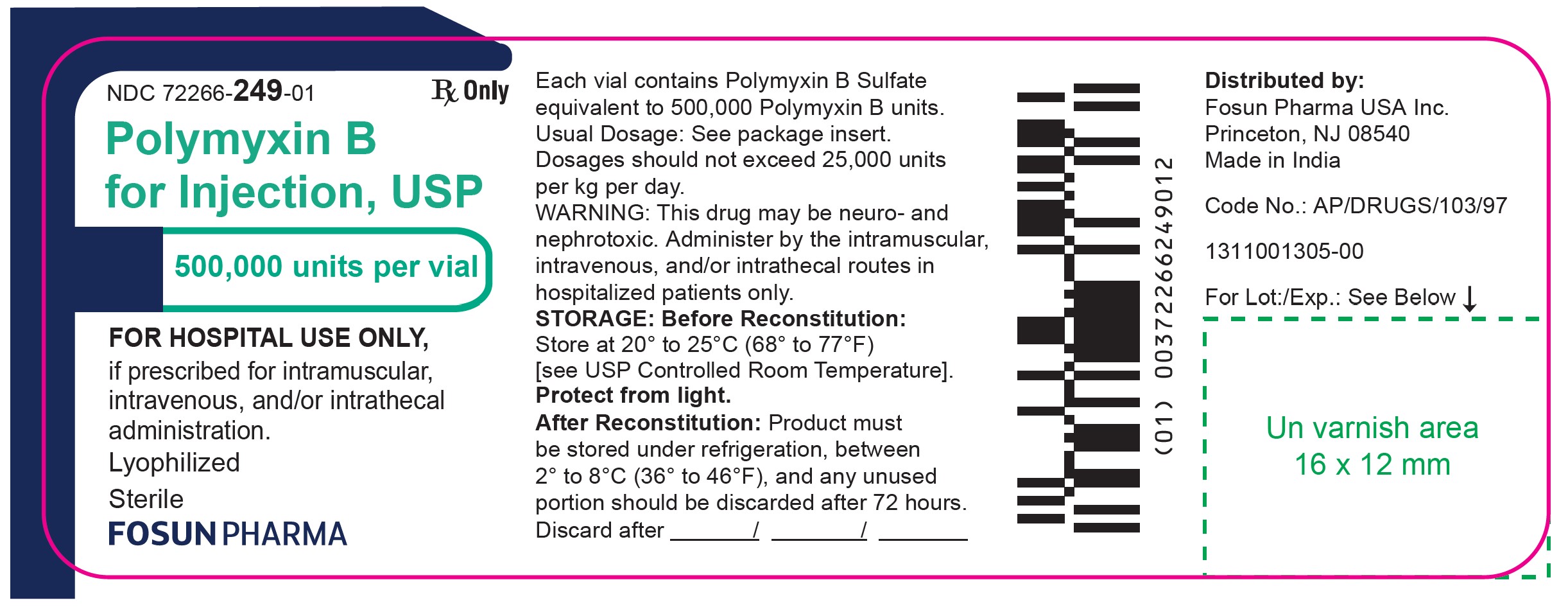
Label: POLYMYXIN B- polymyxin b sulfate injection, powder, lyophilized, for solution
- NDC Code(s): 72266-249-01, 72266-249-10
- Packager: FOSUN PHARMA USA INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
CAUTION: WHEN THIS DRUG IS GIVEN INTRAMUSCULARLY AND/OR INTRATHECALLY, IT SHOULD BE GIVEN ONLY TO HOSPITALIZED PATIENTS, SO AS TO PROVIDE CONSTANT SUPERVISION BY A PHYSICIAN.
RENAL FUNCTION SHOULD BE CAREFULLY DETERMINED AND PATIENTS WITH RENAL DAMAGE AND NITROGEN RETENTION SHOULD HAVE REDUCED DOSAGE. PATIENTS WITH NEPHROTOXICITY DUE TO POLYMYXIN B SULFATE USUALLY SHOW ALBUMINURIA, CELLULAR CASTS, AND AZOTEMIA. DIMINISHING URINE OUTPUT AND A RISING BUN ARE INDICATIONS FOR DISCONTINUING THERAPY WITH THIS DRUG.
NEUROTOXIC REACTIONS MAY BE MANIFESTED BY IRRITABILITY, WEAKNESS, DROWSINESS, ATAXIA, PERIORAL PARESTHESIA, NUMBNESS OF THE EXTREMITIES, AND BLURRING OF VISION. THESE ARE USUALLY ASSOCIATED WITH HIGH SERUM LEVELS FOUND IN PATIENTS WITH IMPAIRED RENAL FUNCTION AND/OR NEPHROTOXICITY.
THE CONCURRENT OR SEQUENTIAL USE OF OTHER NEUROTOXIC AND/OR NEPHROTOXIC DRUGS WITH POLYMYXIN B SULFATE, PARTICULARLY BACITRACIN, STREPTOMYCIN, NEOMYCIN, KANAMYCIN, GENTAMICIN, TOBRAMYCIN, AMIKACIN, CEPHALORIDINE, PAROMOMYCIN, VIOMYCIN, AND COLISTIN SHOULD BE AVOIDED.
THE NEUROTOXICITY OF POLYMYXIN B SULFATE CAN RESULT IN RESPIRATORY PARALYSIS FROM NEUROMUSCULAR BLOCKADE, ESPECIALLY WHEN THE DRUG IS GIVEN SOON AFTER ANESTHESIA AND/OR MUSCLE RELAXANTS.
USAGE IN PREGNANCY: THE SAFETY OF THIS DRUG IN HUMAN PREGNANCY HAS NOT BEEN ESTABLISHED.
-
DESCRIPTION
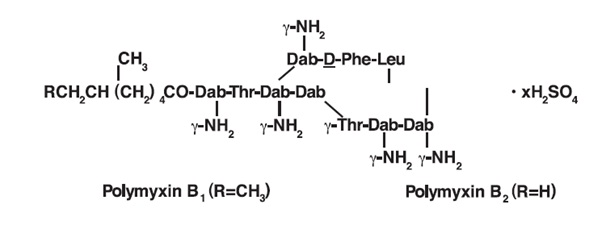
Polymyxin B for Injection, USP is one of a group of basic polypeptide antibiotics derived from B. polymyxa (B. aerosporous). Polymyxin B sulfate is the sulfate salt of Polymyxins B 1 and B 2, which are produced by the growth of Bacillus polymyxa (Prazmowski) Migula (Fam. Bacillacea). It has a potency of not less than 6,000 polymyxin B units per mg, calculated on the anhydrous basis. The structural formulae are:
Each vial contains 500,000 polymyxin B units for parenteral or ophthalmic administration.Polymyxin B for Injection, USP is in powder form suitable for preparation of sterile solutions for intramuscular, intravenous drip, intrathecal, or ophthalmic use.
In the medical literature, dosages have frequently been given in terms of equivalent weights of pure polymyxin B base. Each milligram of pure polymyxin B base is equivalent to 10,000 units of polymyxin B and each microgram of pure polymyxin B base is equivalent to 10 units of polymyxin B.
Aqueous solutions of polymyxin B sulfate may be stored up to 12 months without significant loss of potency if kept under refrigeration. In the interest of safety, solutions for parenteral use should be stored under refrigeration and any unused portion should be discarded after 72 hours. Polymyxin B sulfate should not be stored in alkaline solutions since they are less stable.
-
CLINICAL PHARMACOLOGY
Polymyxin B sulfate has a bactericidal action against almost all gram-negative bacilli except the Proteus group. Polymyxins increase the permeability of the bacterial cell membrane leading to death of the cell. All gram-positive bacteria, fungi, and gram-negative cocci are resistant to polymyxin B.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Polymyxin B sulfate is not absorbed from the normal alimentary tract. Since the drug loses 50 percent of its activity in the presence of serum, active blood levels are low. Repeated injections may give a cumulative effect. Levels tend to be higher in infants and children. The drug is excreted slowly by the kidneys. Tissue diffusion is poor and the drug does not pass the blood brain barrier into the cerebrospinal fluid. In therapeutic dosage, polymyxin B sulfate causes some nephrotoxicity with tubule damage to a slight degree. -
INDICATIONS AND USAGE
Acute Infections Caused by Susceptible Strains of Pseudomonas aeruginosa
Polymyxin B sulfate is a drug of choice in the treatment of infections of the urinary tract, meninges, and bloodstream caused by susceptible strains of Ps. aeruginosa. It may also be used topically and subconjunctivally in the treatment of infections of the eye caused by susceptible strains of Ps. aeruginosa.
It may be indicated in serious infections caused by susceptible strains of the following organisms, when less potentially toxic drugs are ineffective or contraindicated:
H. influenzae, specifically meningeal infections.
Escherichia coli, specifically urinary tract infections.
Aerobacter aerogenes, specifically bacteremia.
Klebsiella pneumoniae, specifically bacteremia.
NOTE: IN MENINGEAL INFECTIONS, POLYMYXIN B SULFATE SHOULD BE ADMINISTERED ONLY BY THE INTRATHECAL ROUTE.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of polymyxin B and other antibacterial drugs, polymyxin B should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- CONTRAINDICATIONS
-
WARNINGS
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Polymyxin B for Injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
General
Prescribing polymyxin B in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
See WARNING box.
Baseline renal function should be done prior to therapy, with frequent monitoring of renal function and blood levels of the drug during parenteral therapy.
Avoid concurrent use of a curariform muscle relaxant and other neurotoxic drugs (ether, tubocurarine, succinylcholine, gallamine, decamethonium and sodium citrate) which may precipitate respiratory depression. If signs of respiratory paralysis appear, respiration should be assisted as required, and the drug discontinued.
As with other antibiotics, use of this drug may result in overgrowth of nonsusceptible organisms, including fungi.
If superinfection occurs, appropriate therapy should be instituted.Information for Patients
Patients should be counseled that antibacterial drugs including polymyxin B should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When polymyxin B is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by polymyxin B or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible. -
ADVERSE REACTIONS
See WARNING box.
Nephrotoxic reactions
Albuminuria, cylinduria, azotemia, and rising blood levels without any increase in dosage.
Neurotoxic reactions
Facial flushing, dizziness progressing to ataxia, drowsiness, peripheral paresthesias (circumoral and stocking glove), apnea due to concurrent use of curariform muscle relaxants, other neurotoxic drugs or inadvertent overdosage, and signs of meningeal irritation with intrathecal administration, e.g., fever, headache, stiff neck and increased cell count and protein cerebrospinal fluid.
Other reactions occasionally reported
Drug fever, urticarial rash, pain (severe) at intramuscular injection sites, and thrombophlebitis at intravenous injection sites.
To report SUSPECTED ADVERSE REACTIONS, contact Fosun Pharma USA Inc. at 1-866-611-3762 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
PARENTERAL
Intravenous
Dissolve 500,000 polymyxin B units in 300 to 500 mL solutions for parenteral dextrose injection 5% for continuous drip.
Adults and children
15,000 to 25,000 units/kg body weight/day in individuals with normal kidney function. This amount should be reduced from 15,000 units/kg downward for individuals with kidney impairment. Infusions may be given every 12 hours, however, the total daily dose must not exceed 25,000 units/kg/day.
Infants
Infants with normal kidney function may receive up to 40,000 units/kg/day without adverse effects.
Intramuscular
Not recommended routinely because of severe pain at injection sites, particularly in infants and children. Dissolve 500,000 polymyxin B units in 2 mL sterile water for injection or sodium chloride injection or procaine hydrochloride injection 1%.
Adults and children
25,000 to 30,000 units/kg/day. This should be reduced in the presence of renal impairment. The dosage may be divided and given at either 4 or 6 hour intervals.
Infants
Infants with normal kidney function may receive up to 40,000 units/kg/day without adverse effects.
Note: Doses as high as 45,000 units/kg/day have been used in limited clinical studies in treating prematures and newborn infants for sepsis caused by Ps. aeruginosa.
Intrathecal
A treatment of choice for Ps aeruginosa meningitis
Dissolve 500,000 polymyxin B units in 10 mL sodium chloride injection USP for 50,000 units per mL dosage unit.
Adults and children over 2 years of age
Dosage is 50,000 units once daily intrathecally for
3 to 4 days, then 50,000 units once every other day for at least 2 weeks after cultures of the cerebrospinal fluid are negative and sugar content has returned to normal.Children under 2 years of age
20,000 units once daily, intrathecally for 3 to 4 days or 25,000 units once every other day. Continue with a dose of 25,000 units once every other day for at least 2 weeks after cultures of the cerebrospinal fluid are negative and sugar content has returned to normal.
IN THE INTEREST OF SAFETY, SOLUTIONS OF PARENTERAL USE SHOULD BE STORED UNDER REFRIGERATION, AND ANY UNUSED PORTIONS SHOULD BE DISCARDED AFTER 72 HOURS.
TOPICAL
Ophthalmic
Dissolve 500,000 polymyxin B units in 20 to 50 mL sterile water for injection or sodium chloride injection USP for a 10,000 to 25,000 units per mL concentration.
For the treatment of Ps. aeruginosa infections of the eye, a concentration of 0.1 percent to 0.25 percent (10,000 units to 25,000 units per mL) is administered 1 to 3 drops every hour, increasing the intervals as response indicates.
Subconjunctival injection of up to 100,000 units/day may be used for the treatment of Ps. aeruginosa infections of the cornea and conjunctiva.
Note: Avoid total systemic and ophthalmic instillation over 25,000 units/kg/day.
-
HOW SUPPLIED
Polymyxin B for Injection, USP is supplied as follows:
NDCPolymyxin B for Injection, USP Package Factor
72266-249-10 500,000 units per vial 10 vials per cartonStorage Conditions
Before Reconstitution: Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]Protect from light. Retain in carton until time of use.
After Reconstitution: Product must be stored under refrigeration, between 2° and 8°C (36° and 46°F), and any unused portion should be discarded after 72 hours.
Lyophilized.
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex.
Distributed by:
Fosun Pharma USA Inc.
Princeton, NJ 08540
Made in India
May 20231313000846-01
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Vial Label
NDC 72266-249-01 Rx only
Polymyxin B for Injection USP
500,000 units per vial
FOR HOSPITAL USE ONLY,
if prescribed for intramuscular,
intravenous, and/or intrathecal
administration.
Sterile Lyophilized
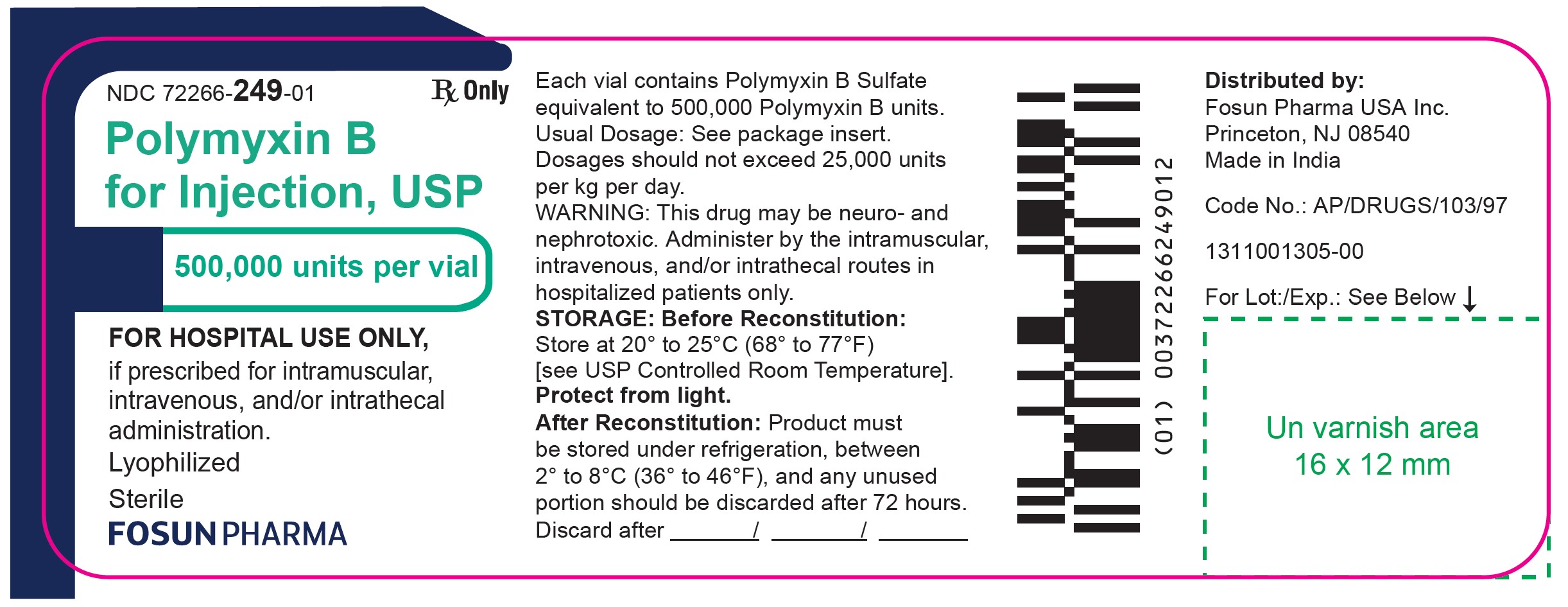
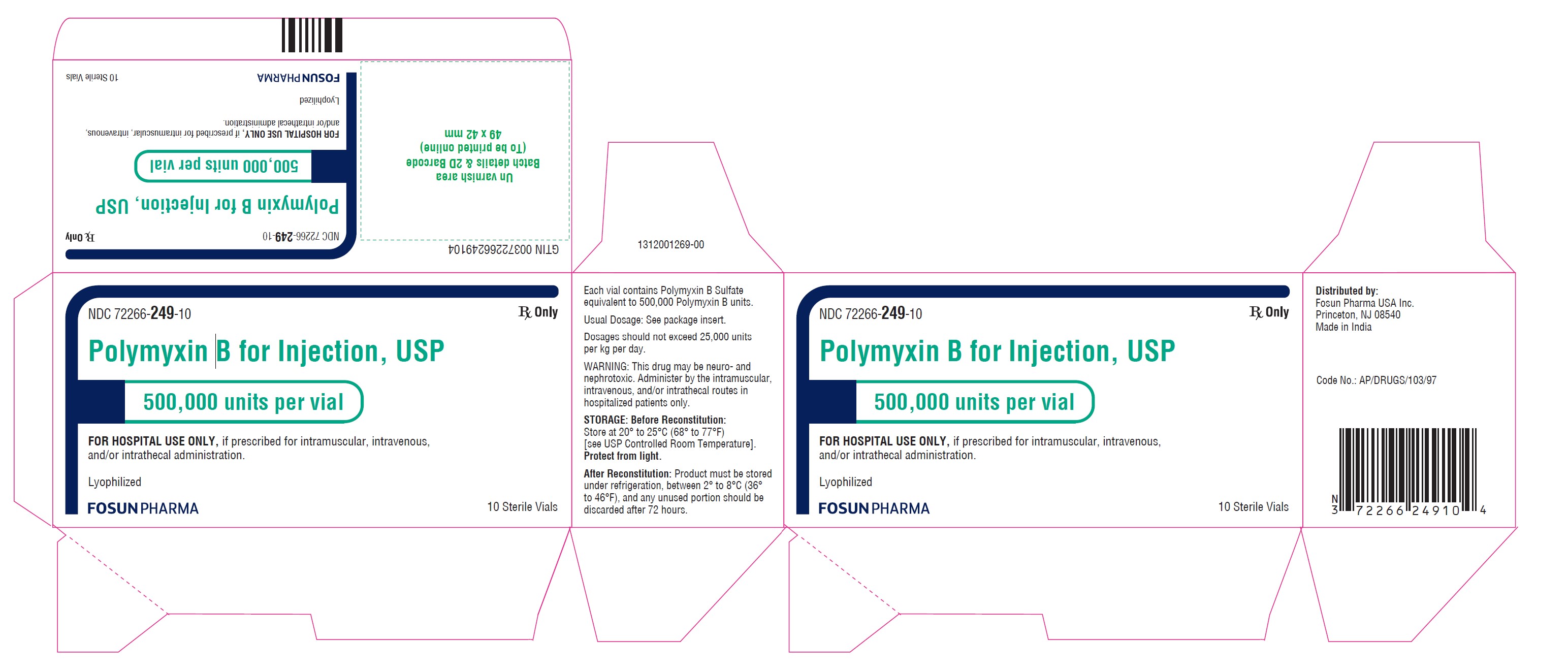
Carton Label
NDC 72266-249-10 10 Sterile vials
Polymyxin B for Injection USP
500,000 units per vial
FOR HOSPITAL USE ONLY,
if prescribed for intramuscular,
intravenous, and/or intrathecal
administration.Rx ONLY
Sterile Lyophilized
-
INGREDIENTS AND APPEARANCE
POLYMYXIN B
polymyxin b sulfate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72266-249 Route of Administration INTRAVENOUS, INTRAMUSCULAR, INTRATHECAL, OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 500000 [USP'U] Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72266-249-10 10 in 1 CARTON 08/31/2023 1 NDC:72266-249-01 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207322 08/31/2023 Labeler - FOSUN PHARMA USA INC (080920998)