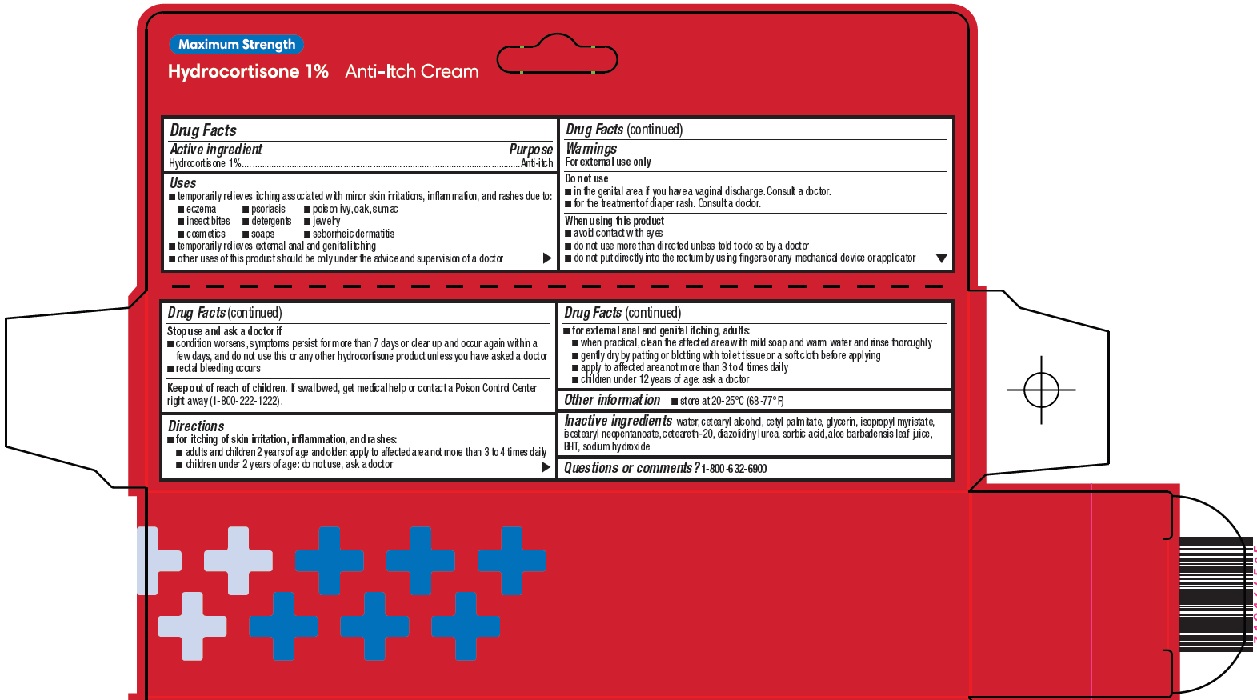
Label: HYDROCORTISONE cream
- NDC Code(s): 30142-411-16
- Packager: Kroger Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
-
Uses
- •
- temporarily relieves itching associated with minor skin irritations, inflammation, and rashes due to:
- •
- eczema
- •
- psoriasis
- •
- poison ivy, oak, sumac
- •
- insect bites
- •
- detergents
- •
- jewelry
- •
- cosmetics
- •
- soaps
- •
- seborrheic dermatitis
- •
- temporarily relieves external anal and genital itching
- •
- other uses of this product should only be under the advice and supervision of a doctor
-
Warnings
For external use only
Do not use
- •
- in the genital area if you have a vaginal discharge. Consult a doctor.
- •
- for the treatment of diaper rash. Consult a doctor.
When using this product
- •
- avoid contact with eyes
- •
- do not use more than directed unless told to do so by a doctor
- •
- do not put directly into the rectum by using fingers or any mechanical device or applicator
-
Directions
- •
- for itching of skin irritation, inflammation, and rashes:
- •
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- •
- children under 2 years of age: do not use, consult ask a doctor
- •
- for external anal and genital itching, adults:
- •
- when practical, clean the affected area with mild soap and warm water and rinse thoroughly
- •
- gently dry by patting or blotting with toilet tissue or a soft cloth before applying
- •
- apply to affected area not more than 3 to 4 times daily
- •
- children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
hydrocortisone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:30142-411 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CETYL PALMITATE (UNII: 5ZA2S6B08X) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOSTEARYL NEOPENTANOATE (UNII: 411THY156Q) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) SORBIC ACID (UNII: X045WJ989B) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:30142-411-16 1 in 1 CARTON 04/13/2017 1 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/13/2017 Labeler - Kroger Company (006999528)