Label: CARPRODYL- carprofen injection, solution
- NDC Code(s): 86136-013-20
- Packager: VetOne
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION(carprofen ...
-
DESCRIPTION:
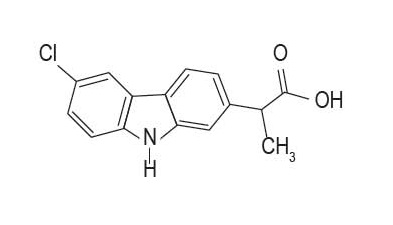
Carprodyl® Injection is a sterile solution containing carprofen, a non-steroidal anti-inflammatory drug (NSAID) of the propionic acid class that includes ibuprofen, naproxen, and ketoprofen ...
-
CLINICAL PHARMACOLOGYCLINICAL PHARMACOLOGY: Carprofen is a non-narcotic, non-steroidal anti-inflammatory agent with characteristic analgesic and antipyretic activity approximately equipotent to indomethacin in animal ...
-
INDICATIONS & USAGEINDICATIONS: Carprodyl® Injection is indicated for the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and ...
-
CONTRAINDICATIONSCONTRAINDICATIONS: Carprodyl® Injection should not be used in dogs exhibiting previous hypersensitivity to carprofen.
-
WARNINGSWARNINGS: Keep out of reach of children. Not for human use. Consult a physician in cases of accidental human exposure.For use in dogs only. Do not use in cats. All dogs should undergo a thorough ...
-
PRECAUTIONSPRECAUTIONS: As a class, cyclooxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Effects may result from decreased prostaglandin production and ...
-
INFORMATION FOR DOG OWNERS:
Carprodyl® Injection, like other drugs of its class, is not free from adverse reactions. Owners should be advised of the potential for adverse reactions and be informed of the clinical signs ...
-
ADVERSE REACTIONSADVERSE REACTIONS: During investigational studies for the caplet formulation, no clinically significant adverse reactions were reported. Some clinical signs were observed during field studies ...
-
DOSAGE & ADMINISTRATIONDOSAGE AND ADMINISTRATION: Carefully consider the potential benefits and risks of carprofen injection and other treatment options before deciding to use carprofen injection. Use the lowest ...
-
SPL UNCLASSIFIED SECTIONEFFECTIVENESS: Confirmation of the effectiveness of carprofen injection for the relief of pain and inflammation associated with osteoarthritis, and for the control of postoperative pain associated ...
-
ANIMAL PHARMACOLOGY & OR TOXICOLOGYANIMAL SAFETY: Laboratory studies in unanesthetized dogs and clinical field studies have demonstrated that carprofen injection is well tolerated in dogs after oral and subcutaneous ...
-
STORAGE AND HANDLINGSTORAGE: Store under refrigeration 2°–8°C (36°–46°F). Once broached, product may be stored at temperatures up to 25°C (77°F) for 28 days.
-
HOW SUPPLIEDHOW SUPPLIED: Carprodyl® Injection is supplied in 20-mL, amber, glass, sterile, multi-dose vials.
-
REFERENCES:
Baruth H, et al: In Anti-Inflammatory and Anti-Rheumatic Drugs, Vol. II, Newer Anti-Inflammatory Drugs, Rainsford KD, ed. CRC Press, Boca Raton, pp. 33–47, 1986. Vane JR, Botting RM: Mechanism ...
-
Principal Display Panel – Vial Label
NDC:86136-013-20 - VETOne - Carprodyl® (carprofen) 50 mg/mL Injection - Non-steroidal anti-inflammatory drug - For subcutaneous use in dogs only - Caution: Federal law restricts this drug to use ...
-
Principal Display Panel – Carton Label
NDC: 86136-013-20 - VETOne - Carprodyl® (carprofen) 50 mg/mL Injection - Non-steroidal anti-inflammatory drug - For subcutaneous use in dogs only - Caution: Federal law restricts this drug to use ...
-
INGREDIENTS AND APPEARANCEProduct Information