Label: WEIGHTLESS SKIN FOUNDATION BROAD SPECTRUM SPF 15- octinoxate, titanium dioxide emulsion
- NDC Code(s): 64141-034-01, 64141-034-02
- Packager: Bobbi Brown Professional Cosmetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS
- PURPOSE
- USE
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive Ingredients
WATER\AQUA\EAU, DIMETHICONE, GLYCERIN, TRIMETHYLSILOXYSILICATE, CETYL PEG/PPG-10/1 DIMETHICONE, BUTYLENE GLYCOL, POLYMETHYLSILSESQUIOXANE, POLYGLYCERYL-3 DIISOSTEARATE, SODIUM CHLORIDE, ACETYL GLUCOSAMINE, DISTEARDIMONIUM HECTORITE, LAMINARIA SACCHARINA EXTRACT, LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL, LAVANDULA HYBRIDA OIL, HONEY EXTRACT\MEL\EXTRAIT DE MIEL, BUTYL AVOCADATE, CAPRYLYL GLYCOL, DISODIUM STEAROYL GLUTAMATE, SODIUM HYALURONATE, SALICYLIC ACID, METHOXY AMODIMETHICONE/SILSESQUIOXANE COPOLYMER, ALUMINUM HYDROXIDE, SILICA, PENTAERYTHRITYL TETRA-DI-T-BUTYL HYDROXYHYDROCINNAMATE, POLYSILICONE-11, CITRIC ACID, SODIUM HYDROXIDE, HEXYLENE GLYCOL, STEARIC ACID, LINALOOL, LINALYL ACETATE, BHT, TOCOPHERYL ACETATE, DISODIUM EDTA, PHENOXYETHANOL, [+/- MICA, TITANIUM DIOXIDE (CI 77891), IRON OXIDES (CI 77491), IRON OXIDES (CI 77492), IRON OXIDES (CI 77499)] <ILN52484>
- Other information
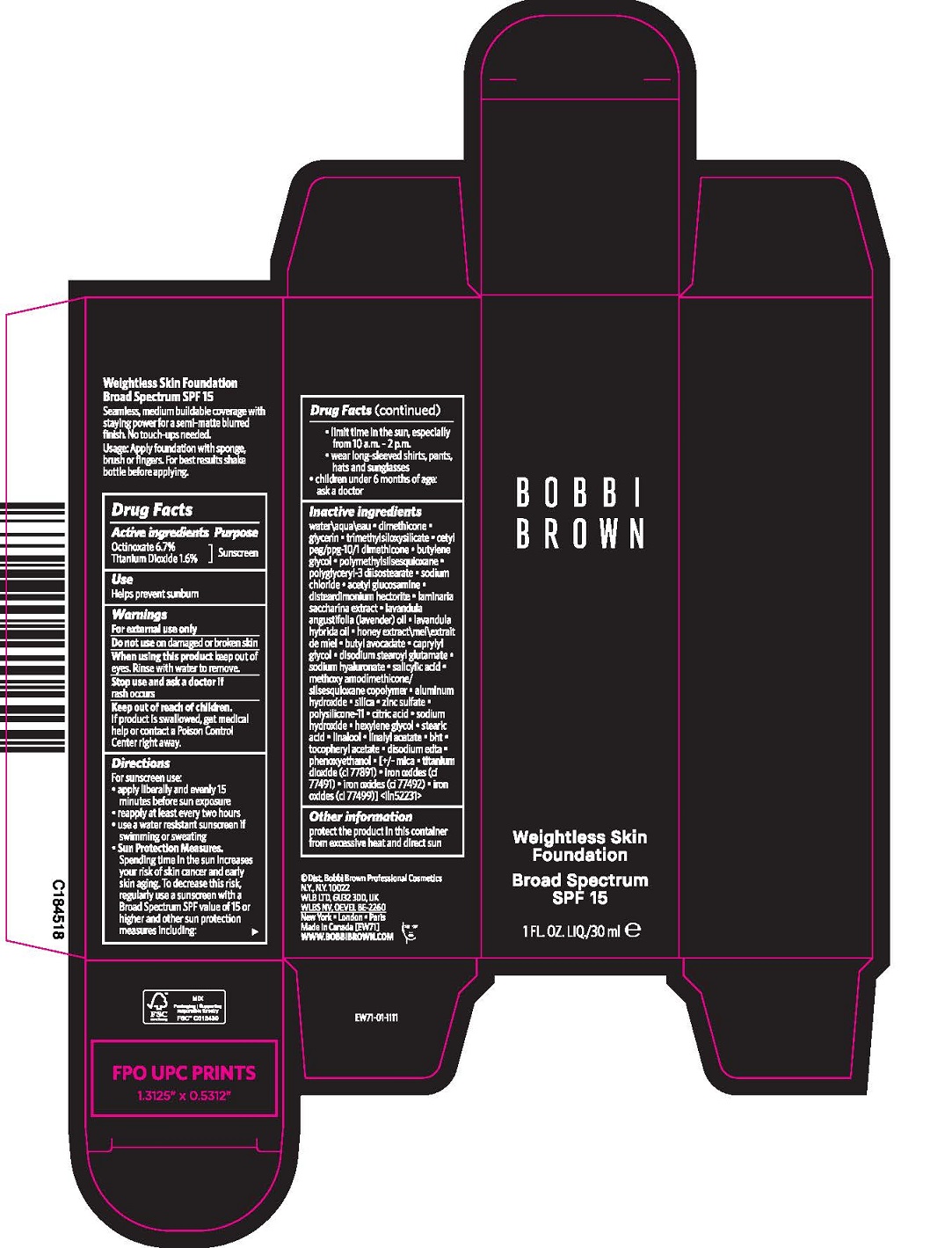
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WEIGHTLESS SKIN FOUNDATION BROAD SPECTRUM SPF 15
octinoxate, titanium dioxide emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64141-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 67 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 17 mg in 1 mL Inactive Ingredients Ingredient Name Strength DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) SACCHARINA LATISSIMA (UNII: 68CMP2MB55) BUTYL AVOCADATE (UNII: Q86RQ0D402) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) HEXYLENE GLYCOL (UNII: KEH0A3F75J) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) WATER (UNII: 059QF0KO0R) LAVANDIN OIL (UNII: 9RES347CKG) SALICYLIC ACID (UNII: O414PZ4LPZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LINALOOL, (+/-)- (UNII: D81QY6I88E) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TRIMETHYLSILOXYSILICATE (M/Q 0.8-1.0) (UNII: 25LXE464L2) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) SODIUM CHLORIDE (UNII: 451W47IQ8X) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 5) (UNII: 035JKJ76MT) N-ACETYLGLUCOSAMINE (UNII: V956696549) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) LINALYL ACETATE (UNII: 5K47SSQ51G) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) DIMETHICONE (UNII: 92RU3N3Y1O) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAVENDER OIL (UNII: ZBP1YXW0H8) HONEY (UNII: Y9H1V576FH) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64141-034-01 1 in 1 CARTON 05/16/2023 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:64141-034-02 1 in 1 CARTON 05/16/2023 2 13 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/16/2023 Labeler - Bobbi Brown Professional Cosmetics Inc. (627131279) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd 202952982 manufacture(64141-034) Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Ltd. 204132062 manufacture(64141-034) , pack(64141-034) , label(64141-034)


 BOBBI
BOBBI