Label: PLUVICTO- lutetium lu 177 vipivotide tetraxetan injection, solution
- NDC Code(s): 69488-010-61
- Packager: Advanced Accelerator Applications USA, Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PLUVICTO safely and effectively. See full prescribing information for PLUVICTO. PLUVICTO® (lutetium Lu 177 vipivotide ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPLUVICTO is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Safety Instructions - PLUVICTO is a radiopharmaceutical; handle with appropriate safety measures to minimize radiation exposure [see Warnings and Precautions (5.1)]. Use ...
-
3 DOSAGE FORMS AND STRENGTHSInjection: 1,000 MBq/mL (27 mCi/mL) of lutetium Lu 177 vipivotide tetraxetan as a clear and colorless to slightly yellow solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Risk From Radiation Exposure - PLUVICTO contributes to a patient’s overall long-term cumulative radiation exposure. Long-term cumulative radiation exposure is associated with an increased ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Myelosuppression [see Warnings and Precautions (5.2)] Renal Toxicity [see Warnings and Precautions ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The safety and efficacy of PLUVICTO have not been established in females. Based on its mechanism of action, PLUVICTO can cause fetal harm [see ...
-
10 OVERDOSAGEIn the event of administration of a radiation overdosage with PLUVICTO, reduce the radiation absorbed dose to the patient by increasing the elimination of the radionuclide from the body by ...
-
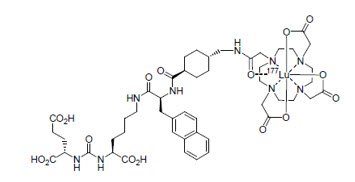
11 DESCRIPTIONPLUVICTO (lutetium Lu 177 vipivotide tetraxetan) is a radioligand therapeutic agent. Lutetium Lu 177 vipivotide tetraxetan is a PSMA-binding ligand bound to a DOTA chelator radiolabeled with ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Lutetium Lu 177 vipivotide tetraxetan is a radioligand therapeutic agent. The active moiety of lutetium Lu 177 vipivotide tetraxetan is the radionuclide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity and mutagenicity studies have not been conducted with lutetium Lu 177 vipivotide tetraxetan; however, radiation is ...
-
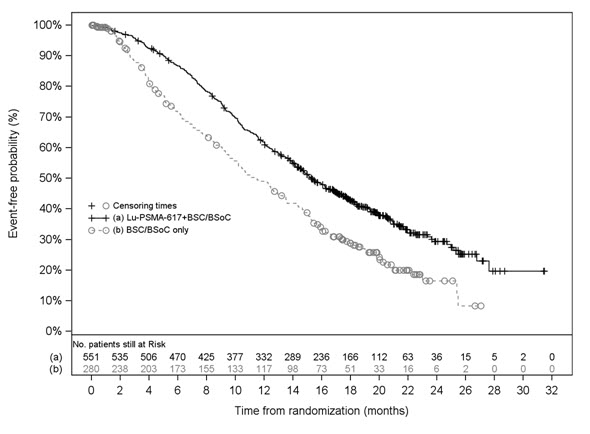
14 CLINICAL STUDIES14.1 PSMA-Positive mCRPC Previously Treated With ARPI Therapy - PSMAfore - The efficacy of PLUVICTO was evaluated in PSMAfore (NCT04689828), a randomized (1:1), multicenter, open-label trial ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPLUVICTO Injection containing 1,000 MBq/mL (27 mCi/mL) of lutetium Lu 177 vipivotide tetraxetan is a sterile, preservative-free and clear, colorless to slightly yellow solution for intravenous use ...
-
17 PATIENT COUNSELING INFORMATIONRisk From Radiation Exposure - Ensure patients increase oral fluid intake and advise patients to void as often as possible to reduce bladder radiation. Before the patient is released, inform ...
-
PRINCIPAL DISPLAY PANELPLUVICTO® 1,000 MBq/mL (27 mCi/mL) lutetium Lu 177 vipivotide tetraxetan injection - Intravenous use Single-dose vial - Sterile - NDC# 69488-010-61 - Rx Only - Advanced Accelerator Applications USA, Inc ...
-
INGREDIENTS AND APPEARANCEProduct Information