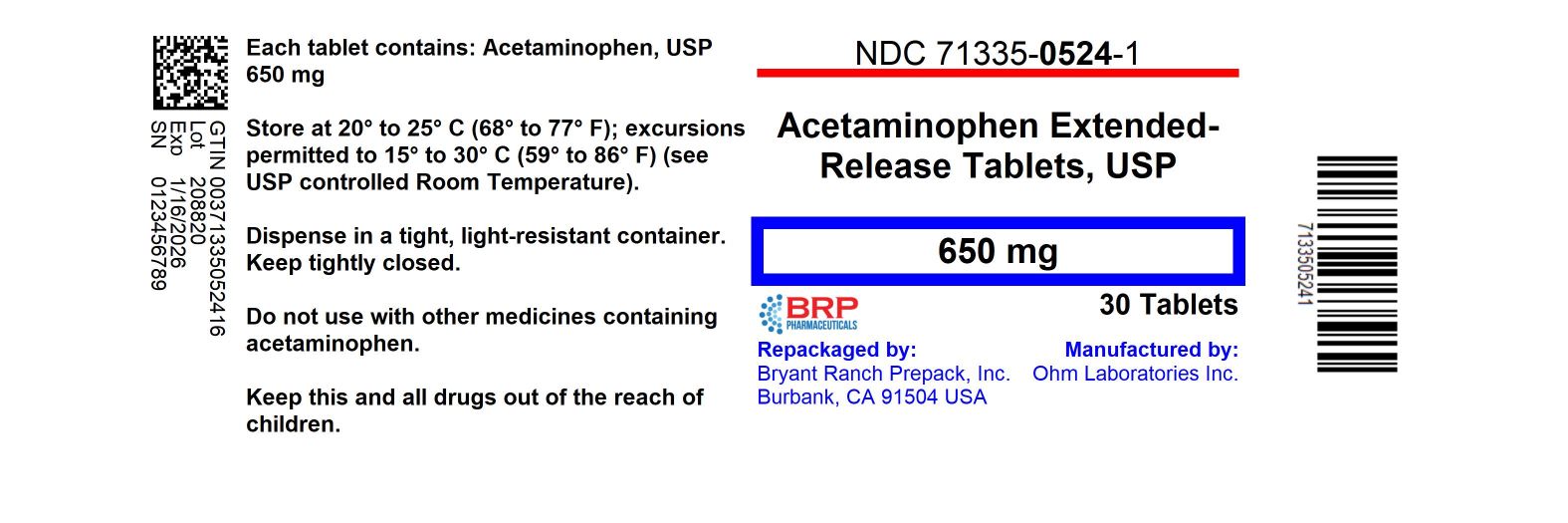
Label: ARTHRITIS PAIN RELIEVER- acetaminophen tablet, film coated, extended release
- NDC Code(s): 71335-0524-1, 71335-0524-2, 71335-0524-3, 71335-0524-4, view more
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 51660-333
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient (in each caplet)Acetaminophen USP, 650 mg
-
PurposePain reliever/fever reducer
-
Usestemporarily relieves minor aches and pains due to: minor pain of arthritis - muscular aches - backache - premenstrual and menstrual cramps - the common cold - headache - toothache - temporarily ...
-
WarningsLiver warning - This product contains acetaminophen. Severe liver damage may occur if you take - more than 6 caplets in 24 hours, which is the maximum daily amount - with other drugs containing ...
-
Directionsdo not take more than directed (see - overdose warning) adults - take 2 caplets every 8 hours with water - swallow whole; do not crush, chew, split or dissolve - do not take ...
-
Other informationstore at 20 - 25° C (68 - 77° F). Avoid excessive heat 40° C (104° F). see end panel for batch number and expiration date - TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL IS BROKEN OR MISSING FROM ...
-
Inactive ingredientscrospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, propylene glycol, sodium lauryl sulfate, stearic acid, titanium dioxide
-
Questions?call - 1-800-406-7984
-
SPL UNCLASSIFIED SECTIONDistributed by: Ohm Laboratories Inc. New Brunswick, NJ 08901
-
HOW SUPPLIED
NDC: 71335-0524-1: 30 Tablets in a BOTTLE - NDC: 71335-0524-2: 100 Tablets in a BOTTLE - NDC: 71335-0524-3: 50 Tablets in a BOTTLE - NDC: 71335-0524-4: 60 Tablets in a BOTTLE - NDC: 71335-0524-6: 19 ...
-
PRINCIPAL DISPLAY PANELAcetaminophen ER 650mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information