[See FDA-approved patient labeling (Patient Information)]
Patients taking Minocycline hydrochloride extended-release tablets should receive the following information and ...
[See FDA-approved patient labeling (Patient Information)]
Patients taking Minocycline hydrochloride extended-release tablets should receive the following information and instructions:
- Minocycline hydrochloride extended-release tablets should not be used by pregnant women or women attempting to conceive a child [see Use in Specific Populations (8.1), Nonclinical Toxicology (13.1)].
- It is recommended that Minocycline hydrochloride extended-release tablets not be used by men who are attempting to father a child [see Nonclinical Toxicology (13.1)].
- Patients should be advised that pseudomembranous colitis can occur with minocycline therapy. If patients develop watery or bloody stools, they should seek medical attention.
- Patients should be counseled about the possibility of hepatotoxicity. Patients should seek medical advice if they experience symptoms which can include loss of appetite, tiredness, diarrhea, skin turning yellow, bleeding easily, confusion, and sleepiness.
- Patients who experience central nervous system symptoms [see Warnings and Precautions (5.5)] should be cautioned about driving vehicles or using hazardous machinery while on minocycline therapy. Patients should seek medical help for persistent headaches or blurred vision.
- Concurrent use of tetracycline may render oral contraceptives less effective [see Drug Interactions (7.5)].
- Autoimmune syndromes, including drug-induced lupus-like syndrome, autoimmune hepatitis, vasculitis and serum sickness have been observed with tetracycline-class drugs, including minocycline. Symptoms may be manifested by arthralgia, fever, rash and malaise. Patients who experience such symptoms should be cautioned to stop the drug immediately and seek medical help.
- Patients should be counseled about discoloration of skin, scars, teeth or gums that can arise from minocycline therapy.
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including minocycline. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while using minocycline. If patients need to be outdoors while using minocycline, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. Treatment should be discontinued at the first evidence of skin erythema.
- Minocycline hydrochloride extended-release tablets should be taken exactly as directed. Skipping doses or not completing the full course of therapy may decrease the effectiveness of the current treatment course and increase the likelihood that bacteria will develop resistance and will not be treatable by other antibacterial drugs in the future.
- Patients should be advised to swallow Minocycline hydrochloride extended-release tablets whole and not to chew, crush, or split the tablets.
Manufactured by:
Alkem Laboratories Limited,
Mumbai - 400 013, INDIA
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Patient Information
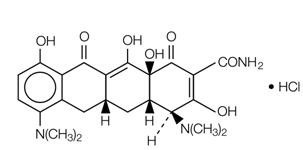
Minocycline hydrochloride extended-release tablets
Rx only
Read this Patient information Leaflet that comes with Minocycline hydrochloride extended-release tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition or treatment.
What is Minocycline hydrochloride extended-release tablets?
Minocycline hydrochloride extended-release tablets is a tetracycline-class drug. Minocycline hydrochloride extended-release tablets is prescription medicine used to treat pimples and red bumps (non-nodular inflammatory lesions) that happen with moderate to severe acne vulgaris in people 12 years and older. Minocycline hydrochloride extended-release tablets is not effective for acne that is not red-looking (this means acne that is not inflammatory).
It is not known if Minocycline hydrochloride extended-release tablets is:
- safe for use longer than 12 weeks.
- safe and effective for the treatment of infections.
- safe and effective in children under the age of 12 years.
Who should not take Minocycline hydrochloride extended-release tablets?
Do not take Minocycline hydrochloride extended-release tablets if you are allergic to tetracycline-class drugs. Ask your doctor or pharmacist for a list of these medicines if you are not sure.
What should I tell my doctor before taking Minocycline hydrochloride extended-release tablets?
Before you take Minocycline hydrochloride extended-release tablets tell your doctor if you:
- have kidney problems. Your doctor may prescribe a lower dose of medicine for you.
- have liver problems.
- have diarrhea or watery stools.
- have vision problems.
- plan to have surgery with general anesthesia.
- have any other medical conditions.
- are a male, and you and your female partner are trying to conceive a baby. You should not take Minocycline hydrochloride extended-release tablets.
- are pregnant or plan to become pregnant. Minocycline hydrochloride extended- release tablets may harm your unborn baby. Taking Minocycline hydrochloride extended-release tablets while you are pregnant may cause serious side effects on the growth of bone and teeth of your baby. Talk to your doctor before taking Minocycline hydrochloride extended- release tablets if you plan to become pregnant, or if you are already taking Minocycline hydrochloride extended-release tablets and plan to become pregnant. Stop taking Minocycline hydrochloride extended-release tablets and call your doctor right away if you become pregnant while taking Minocycline hydrochloride extended-release tablets.
- are breastfeeding or plan to breastfeed. Minocycline hydrochloride passes into your milk and may harm your baby. You and your doctor should decide if you will take Minocycline hydrochloride extended-release tablets or breastfeed. You should not do both.
Tell your doctor about all the other medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Minocycline hydrochloride extended-release tablets may affect the way other medicines work, and other medicines may affect how Minocycline hydrochloride extended-release tablets works.
Especially tell your doctor if you take:
-
birth control pills. Minocycline hydrochloride extended-release tablets may make your birth control pills less effective. You could become pregnant. You should use a second form of birth control while taking Minocycline hydrochloride extended-release tablets.
-
a blood thinner medicine.
-
a penicillin antibiotic medicine. Minocycline hydrochloride extended-release tablets and penicillins should not be used together.
-
antacids that contain aluminum, calcium, or magnesium or iron-containing products.
- an acne medicine that contains isotretinoin (Amnesteem, Claravis, Sotret). Minocycline hydrochloride extended-release tablets and isotretinoin should not be used together.
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist.
How should I take Minocycline hydrochloride extended-release tablets?
- Take Minocycline hydrochloride extended-release tablets exactly as your doctor tells you.
- Skipping doses or not taking all doses of Minocycline hydrochloride extended-release tablets may:
- make the treatment not work as well.
- increase the chance that the bacteria will become resistant to Minocycline hydrochloride extended-release tablets.
-
Minocycline hydrochloride extended-release tablets can be taken with or without food. Taking Minocycline hydrochloride extended-release tablets with food may lower your chances of getting irritation or ulcers in your esophagus. Your esophagus is the tube that connects your mouth to your stomach.
-
Swallow Minocycline hydrochloride extended-release tablets whole. Do not chew, crush, or split the tablets.
If you take too much Minocycline hydrochloride extended-release tablets, call your doctor or poison control center right away. Your doctor may do blood tests to check you for side effects during treatment with Minocycline hydrochloride extended-release tablets.
What should I avoid while taking Minocycline hydrochloride extended-release tablets?
- Avoid sunlight, sunlamps, and tanning beds. Minocycline hydrochloride extended-release tablets can make your skin sensitive to the sun and the light from sunlamps and tanning beds. You could get severe sunburn.
- Protect your skin while out in sunlight.
- You should not drive or operate dangerous machinery until you know how Minocycline hydrochloride extended-release tablets affects you. Minocycline hydrochloride extended-release tablets may cause you to feel dizzy or lightheaded, or have a spinning feeling (vertigo).
What are possible side effects of Minocycline hydrochloride extended-release tablets?
Minocycline hydrochloride extended-release tablets may cause serious side effects, including:
-
Harm to an unborn baby. See "What should I tell my doctor before taking Minocycline hydrochloride extended-release tablets?"
-
Permanent teeth discoloration. Minocycline hydrochloride extended-release tablets may permanently turn a baby or child's teeth yellow-grey-brown during tooth development. Minocycline hydrochloride extended-release tablets should not be used during tooth development. Tooth development happens in the last half of pregnancy, and from birth to 8 years of age. See "What should I tell my doctor before taking Minocycline hydrochloride extended-release tablets?"
-
Intestine infection (pseudomembranous colitis). Pseudomembranous colitis can happen with most antibiotics, including Minocycline hydrochloride extended-release tablets. Call your doctor right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools.
-
Serious liver problems. Stop taking Minocycline hydrochloride extended-release tablets and call your doctor right away if you get any of the following symptoms of liver problems:
- loss of appetite
- tiredness
- diarrhea
- yellowing of your skin or the whites of your eyes
- unexplained bleeding
- confusion
- sleepiness
-
Central nervous system effects. See "What should I avoid while taking Minocycline hydrochloride extended-release tablets?" Central nervous system effects such as light headedness, dizziness, and a spinning feeling (vertigo) may go away during your treatment with Minocycline hydrochloride extended-release tablets or if treatment is stopped.
-
Benign intracranial hypertension, also called pseudotumor cerebri. This is a condition where there is high pressure in the fluid around the brain. This swelling may lead to vision changes and permanent vision loss. Stop taking Minocycline hydrochloride extended-release tablets and tell your doctor right away if you have blurred vision, vision loss, or unusual headaches. Immune system reactions including a lupus-like syndrome, hepatitis, and inflammation of blood or lymph vessels (vasculitis ). Using Minocycline hydrochloride extended-release tablets for a long time to treat acne may cause immune system reactions. Tell your doctor right away if you get a fever, rash, joint pain, or body weakness. Your doctor may do tests to check your blood for immune system reactions.
-
Serious rash and allergic reactions. Minocycline hydrochloride extended-release tablets may cause a serious rash and allergic reactions that may affect parts of your body such as your liver, lungs, kidneys and heart. Sometimes these can lead to death.
- Stop taking Minocycline hydrochloride extended-release tablets and get medical help right away if you have any of these symptoms:
- skin rash, hives, sores in your mouth, or your skin blisters and peels
- swelling of your face, eyes, lips, tongue, or throat
- trouble swallowing or breathing
- blood in your urine
- fever, yellowing of the skin or the whites of your eyes, dark colored urine
- pain on the right side of the stomach area (abdominal pain)
- chest pain or abnormal heartbeats
- swelling in your legs, ankles, and feet
- darkening of your nails, skin, eyes, scars, teeth, and gums
The most common side effects of Minocycline hydrochloride extended-release tablets include:
- headache
- tiredness
- dizziness or spinning feeling
- itching
Call your doctor if you have a side effect that bothers you or that does not go away. Your doctor may do tests to check you for side effects during treatment with Minocycline hydrochloride extended-release tablets.
These are not all the side effects with Minocycline hydrochloride extended-release tablets. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store minocycline hydrochloride extended-release tablets?
- Store minocycline hydrochloride extended-release tablets between 68°F to 77°F (20°C to 25°C).
- Keep Minocycline hydrochloride extended-release tablets in the container that it comes in and keep the container tightly closed.
- Keep Minocycline hydrochloride extended-release tablets dry.
Keep Minocycline hydrochloride extended-release tablets and all medicines out of the reach of children.
General information about Minocycline hydrochloride extended-release tablets
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Minocycline hydrochloride extended-release tablets for a condition for which it was not prescribed. Do not give Minocycline hydrochloride extended-release tablets to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Minocycline hydrochloride extended-release tablets. If you would like more information, talk to your doctor. You can ask your doctor or pharmacist for information about Minocycline hydrochloride extended-release tablets that is written for health professionals.
For more information, call 1-877-ASC-RX01 (877-272-7901).
What are the ingredients in Minocycline hydrochloride extended-release tablets?
Active ingredient: minocycline HCl.
Inactive ingredients: lactose monohydrate, hypromellose type 2910, hypromellose type 2208, colloidal silicon dioxide, magnesium stearate, titanium dioxide and triacetin.
The 45 mg tablets also contain iron oxide black.
The 65 mg tablets also contain FD&C blue #1/brilliant blue FCF aluminium lake, polyethylene glycol 3350, FD&C blue #2/indigo carmine aluminum lake and D&C yellow #10 aluminum lake.
The 55 mg tablets also contain macrogol, FD&C RED #40.
The 80 mg tablets also contain macrogol, FD&C blue #2, FD&C red #40, FD&C yellow #6.
The 90 mg tablets also contain iron oxide yellow and polyethylene glycol 3350.
The 105 mg tablets also D&C red #27, macrogol, FD&C blue #1.
The 115 mg tablets also contain D&C yellow #10 aluminum lake, FD&C blue #1/brilliant blue FCF aluminium lake and FD&C blue #2/indigo carmine aluminum lake.
The 135 mg tablets also contain polyethylene glycol 3350 and iron oxide red.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Alkem Laboratories Limited,
Mumbai - 400 013, INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised: September,2019
PT 3278-01
Close