Label: CEFPODOXIME PROXETIL tablet, film coated
- NDC Code(s): 51407-083-20, 51407-084-20
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 67877-878, 67877-879
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
Cefpodoxime Proxetil Tablets, USP
Rx only
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil tablets, USP and other antibacterial drugs, cefpodoxime proxetil tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
For Oral Use Only -
DESCRIPTION
Cefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R, 7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z) methoxyimino} acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene-2-carboxylate.
Its empirical formula is C 21H 27N 5O 9S 2 and its structural formula is represented below:
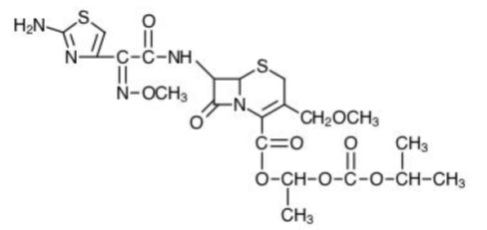
The molecular weight of cefpodoxime proxetil is 557.6.
Cefpodoxime proxetil is a prodrug; its active metabolite is cefpodoxime. All doses of cefpodoxime proxetil in this insert are expressed in terms of the active cefpodoxime moiety. The drug is supplied as film-coated tablets.
Cefpodoxime proxetil tablets, USP contain cefpodoxime proxetil USP equivalent to 100 mg or 200 mg of cefpodoxime activity. Each film-coated tablet contains the following inactive ingredients:
Carboxymethyl cellulose calcium, colloidal silicon dioxide, crospovidone, FD&C Yellow No. 6, hydroxypropyl cellulose, hypromellose, iron oxide red, lactose monohydrate, macrogol, magnesium stearate, sodium lauryl sulfate and titanium dioxide. -
CLINICAL PHARMACOLOGY
Absorption and Excretion
Cefpodoxime proxetil is a prodrug that is absorbed from the gastrointestinal tract and de-esterified to its active metabolite, cefpodoxime. Following oral administration of 100 mg of cefpodoxime proxetil to fasting subjects, approximately 50% of the administered cefpodoxime dose was absorbed systemically. Over the recommended dosing range (100 to 400 mg), approximately 29 to 33% of the administered cefpodoxime dose was excreted unchanged in the urine in 12 hours. There is minimal metabolism of cefpodoxime in vivo.
Effects of Food
The extent of absorption (mean AUC) and the mean peak plasma concentration increased when film coated tablets were administered with food. Following a 200 mg tablet dose taken with food, the AUC was 21 to 33% higher than under fasting conditions, and the peak plasma concentration averaged 3.1 mcg/mL in fed subjects versus 2.6 mcg/mL in fasted subjects. Time to peak concentration was not significantly different between fed and fasted subjects.
When a 200 mg dose of the suspension was taken with food, the extent of absorption (mean AUC) and mean peak plasma concentration in fed subjects were not significantly different from fasted subjects, but the rate of absorption was slower with food (48% increase in T max).
Pharmacokinetics of cefpodoxime proxetil film-coated tablets
Over the recommended dosing range (100 to 400 mg), the rate and extent of cefpodoxime absorption exhibited dose-dependency; dose-normalized C max and AUC decreased by up to 32% with increasing dose. Over the recommended dosing range, the T max was approximately 2 to 3 hours and the T1/2 ranged from 2.09 to 2.84 hours. Mean C max was 1.4 mcg/mL for the 100 mg dose, 2.3 mcg/mL for the 200 mg dose, and 3.9 mcg/mL for the 400 mg dose. In patients with normal renal function, neither accumulation nor significant changes in other pharmacokinetic parameters were noted following multiple oral doses of up to 400 mg Q 12 hours.
Cefpodoxime Plasma Levels (mcg/mL) in Fasted Adults After Film-coated Tablet Administration (Single Dose)
Dose
Time after Oral Ingestion
(Cefpodoxime
Equivalents )
1hr
2hr
3hr
4hr
6hr
8hr
12hr
100 mg
0.98
1.4
1.3
1
0.59
0.29
0.08
200 mg
1.5
2.2
2.2
1.8
1.2
0.62
0.18
400 mg
2.2
3.7
3.8
3.3
2.3
1.3
0.38
Distribution
Protein binding of cefpodoxime ranges from 22 to 33% in serum and from 21 to 29% in plasma.
Skin Blister
Following multiple-dose administration every 12 hours for 5 days of 200 mg or 400 mg cefpodoxime proxetil, the mean maximum cefpodoxime concentration in skin blister fluid averaged 1.6 and 2.8 mcg/mL, respectively. Skin blister fluid cefpodoxime levels at 12 hours after dosing averaged 0.2 and 0.4 mcg/mL for the 200 mg and 400 mg multiple-dose regimens, respectively.
Tonsil Tissue
Following a single, oral 100 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in tonsil tissue averaged 0.24 mcg/g at 4 hours post-dosing and 0.09 mcg/g at 7 hours post-dosing. Equilibrium was achieved between plasma and tonsil tissue within 4 hours of dosing. No detection of cefpodoxime in tonsillar tissue was reported 12 hours after dosing. These results demonstrated that concentrations of cefpodoxime exceeded the MIC 90 of S. pyogenes for at least 7 hours after dosing of 100 mg of cefpodoxime proxetil.
Lung Tissue
Following a single, oral 200 mg cefpodoxime proxetil film-coated tablet, the mean maximum cefpodoxime concentration in lung tissue averaged 0.63 mcg/g at 3 hours post-dosing, 0.52 mcg/g at 6 hours postdosing, and 0.19 mcg/g at 12 hours post-dosing. The results of this study indicated that cefpodoxime penetrated into lung tissue and produced sustained drug concentrations for at least 12 hours after dosing at levels that exceeded the MIC 90 for S. pneumoniae and H. influenzae.
CSF
Adequate data on CSF levels of cefpodoxime are not available.
Effects of Decreased Renal Function
Elimination of cefpodoxime is reduced in patients with moderate to severe renal impairment (<50 mL/min creatinine clearance). (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.) In subjects with mild impairment of renal function (50 to 80 mL/min creatinine clearance), the average plasma half-life of cefpodoxime was 3.5 hours. In subjects with moderate (30 to 49 mL/min creatinine clearance) or severe renal impairment (5 to 29 mL/min creatinine clearance), the half-life increased to 5.9 and 9.8 hours, respectively. Approximately 23% of the administered dose was cleared from the body during a standard 3-hour hemodialysis procedure.
Effect of Hepatic Impairment (cirrhosis)
Absorption was somewhat diminished and elimination unchanged in patients with cirrhosis. The mean cefpodoxime T 1/2 and renal clearance in cirrhotic patients were similar to those derived in studies of healthy subjects. Ascites did not appear to affect values in cirrhotic subjects. No dosage adjustment is recommended in this patient population.
Pharmacokinetics in Elderly Subjects
Elderly subjects do not require dosage adjustments unless they have diminished renal function. (See PRECAUTIONS.) In healthy geriatric subjects, cefpodoxime half-life in plasma averaged 4.2 hours (vs 3.3 in younger subjects) and urinary recovery averaged 21% after a 400 mg dose was administered every 12 hours. Other pharmacokinetic parameters (C max, AUC, and T max) were unchanged relative to those observed in healthy young subjects.
Microbiology
Mechanism of Action
Cefpodoxime is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis.
Cefpodoxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Mechanism of Resistance
Resistance to cefpodoxime is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability.
Cefpodoxime has been shown to be active against most isolates of the following bacteria, both in vitro and in clinical infections as described in the Indications and Usage (1) section:
Gram-positive bacteria
Staphylococcus aureus (methicillin-susceptible strains, including those producing penicillinases)
Staphylococcus saprophyticus
Streptococcus pneumoniae (excluding penicillin-resistant isolates)
Streptococcus pyogenes
Gram-negative bacteria
Escherichia coli
Klebsiella pneumoniae
Proteus mirabilis
Haemophilus influenzae (including beta-lactamase producing isolates)
Moraxella catarrhalis
Neisseria gonorrhoeae (including penicillinase-producing isolates)
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for cefpodoxime. However, the efficacy of cefpodoxime in treating clinical infections due to these microorganisms has not been established in adequate and well controlled clinical trials.
Gram-positive bacteria
Streptococcus agalactiae
Streptococcus spp. (Groups C, F, G)
Gram-negative bacteria
Citrobacterdiversus
Klebsiellaoxytoca
Proteus vulgaris
Providenciarettgeri
Haemophilusparainfluenzae
Anaerobic Gram-positive bacteria
Peptostreptococcusmagnus
Susceptibility Test Methods
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC -
INDICATIONS AND USAGE
Cefpodoxime proxetil is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Recommended dosages, durations of therapy, and applicable patient populations vary among these infections. Please see DOSAGE AND ADMINISTRATION for specific recommendations.
Acute otitis media caused by Streptococcus pneumoniae (excluding penicillin-resistant strains), Streptococcus pyogenes, Haemophilusinfluenzae (including beta-lactamase-producing strains), or Moraxella (Branhamella) catarrhalis (including beta-lactamase-producing strains).
Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes.
NOTE: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Cefpodoxime proxetil is generally effective in the eradication of streptococci from the oropharynx. However, data establishing the efficacy of cefpodoxime proxetil for the prophylaxis of subsequent rheumatic fever are not available.
Community-acquired pneumonia caused by S. pneumoniae or H. Influenzae (including beta-lactamase-producing strains).
Acute bacterial exacerbation of chronic bronchitis caused by S. pneumoniae, H. influenzae beta-lactamase-producing strains only), or M. catarrhalis. Data are insufficient at this time to establish efficacy in patients with acute bacterial exacerbations of chronic bronchitis caused by beta-lactamase-producing strains of H. influenzae.
Acute, uncomplicated urethral and cervical gonorrhea caused by Neisseria gonorrhoeae (including penicillinase-producing strains).
Acute, uncomplicated ano-rectal infections in women due to Neisseria gonorrhoeae (including penicillinase-producing strains).
NOTE: The efficacy of cefpodoxime in treating male patients with rectal infections caused by N. gonorrhoeae has not been established. Data do not support the use of cefpodoxime proxetil in the treatment of pharyngeal infections due to N. gonorrhoeae in men or women.
Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (including penicillinase-producing strains) or Streptococcus pyogenes. Abscesses should be surgically drained as clinically indicated.
NOTE: In clinical trials, successful treatment of uncomplicated skin and skin structure infections was dose-related. The effective therapeutic dose for skin infections was higher than those used in other recommended indications. (See DOSAGE AND ADMINISTRATION.)
Acute maxillary sinusitis caused by Haemophilusinfluenzae (including beta-lactamase-producing strains), Streptococcus pneumoniae, and Moraxella catarrhalis.
Uncomplicated urinary tract infections (cystitis) caused by Escherichia coli, Klebsiellapneumoniae, Proteus mirabilis, or Staphylococcus saprophyticus.
NOTE: In considering the use of cefpodoxime proxetil in the treatment of cystitis, cefpodoxime proxetil’s lower bacterial eradication rates should be weighed against the increased eradication rates and different safety profiles of some other classes of approved agents. (See CLINICAL STUDIES section.)
Appropriate specimens for bacteriological examination should be obtained in order to isolate and identify causative organisms and to determine their susceptibility to cefpodoxime. Therapy may be instituted while awaiting the results of these studies. Once these results become available, antimicrobial therapy should be adjusted accordingly.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil tablets, and other antibacterial drugs, cefpodoxime proxetil tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy. - CONTRAINDICATIONS
-
WARNINGS
BEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPODOXIME, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFPODOXIME IS TO BE ADMINISTERED TO PENICILLIN SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPODOXIME PROXETIL OCCURS, DISCONTINUE THE DRUG.
SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINE, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefpodoxime proxetil tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
A concerted effort to monitor for C. difficile in cefpodoxime-treated patients with diarrhea was undertaken because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients.
In post-marketing experience outside the United States, reports of pseudomembranous colitis associated with the use of cefpodoxime proxetil have been received. -
PRECAUTIONS
General
In patients with transient or persistent reduction in urinary output due to renal insufficiency, the total daily dose of cefpodoxime proxetil should be reduced because high and prolonged serum antibiotic concentrations can occur in such individuals following usual doses. Cefpodoxime, like other cephalosporins, should be administered with caution to patients receiving concurrent treatment with potent diuretics. (See DOSAGE AND ADMINISTRATION.)
As with other antibiotics, prolonged use of cefpodoxime proxetil may result in overgrowth of non-susceptible organisms. Repeated evaluation of the patient’s condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Prescribing cefpodoxime proxetil tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Information for Patients
Patients should be counseled that antibacterial drugs including cefpodoxime proxetil tablets, USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefpodoxime proxetil tablets, USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefpodoxime proxetil tablets, USP or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
Antacids
Concomitant administration of high doses of antacids (sodium bicarbonate and aluminum hydroxide) or H 2 blockers reduces peak plasma levels by 24% to 42% and the extent of absorption by 27% to 32%, respectively. The rate of absorption is not altered by these concomitant medications. Oral anti-cholinergics (e.g., propantheline) delay peak plasma levels (47% increase in Tmax), but do not affect the extent of absorption (AUC).
Probenecid
As with other beta-lactam antibiotics, renal excretion of cefpodoxime was inhibited by probenecid and resulted in an approximately 31% increase in AUC and 20% increase in peak cefpodoxime plasma levels.
Nephrotoxic drugs
Although nephrotoxicity has not been noted when cefpodoxime proxetil was given alone, close monitoring of renal function is advised when cefpodoxime proxetil is administered concomitantly with compounds of known nephrotoxic potential.Drug/Laboratory Test Interactions
Cephalosporins, including cefpodoxime proxetil, are known to occasionally induce a positive direct Coombs’ test.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal carcinogenesis studies of cefpodoxime proxetil have not been performed.
Mutagenesis studies of cefpodoxime, including the Ames test both with and without metabolic activation, the chromosome aberration test, the unscheduled DNA synthesis assay, mitotic recombination and gene conversion, the forward gene mutation assay and the in vivo micronucleus test, were all negative. No untoward effects on fertility or reproduction were noted when 100 mg/kg/day or less (2 times the human dose based on mg/m2) was administered orally to rats.
Pregnancy
Teratogenic Effects
Pregnancy Category BCefpodoxime proxetil was neither teratogenic nor embryocidal when administered to rats during organogenesis at doses up to 100 mg/kg/day (2 times the human dose based on mg/m2) or to rabbits at doses up to 30 mg/kg/day (1 to 2 times the human dose based on mg/m2).
There are, however, no adequate and well-controlled studies of cefpodoxime proxetil use in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.Labor and Delivery
Cefpodoxime proxetil has not been studied for use during labor and delivery. Treatment should only be given if clearly needed.
Nursing Mothers
Cefpodoxime is excreted in human milk. In a study of 3 lactating women, levels of cefpodoxime in human milk were 0%, 2% and 6% of concomitant serum levels at 4 hours following a 200 mg oral dose of cefpodoxime proxetil. At 6 hours post-dosing, levels were 0%, 9% and 16% of concomitant serum levels. Because of the potential for serious reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
Of the 3338 patients in multiple-dose clinical studies of cefpodoxime proxetil film-coated tablets, 521 (16%) were 65 and over, while 214 (6%) were 75 and over. No overall differences in effectiveness or safety were observed between the elderly and younger patients. In healthy geriatric subjects with normal renal function, cefpodoxime half-life in plasma averaged 4.2 hours and urinary recovery averaged 21% after a 400 mg dose was given every 12 hours for 15 days. Other pharmacokinetic parameters were unchanged relative to those observed in healthy younger subjects.
Dose adjustment in elderly patients with normal renal function is not necessary.
-
ADVERSE REACTIONS
Clinical Trials
Film-coated Tablets (Multiple dose)
In clinical trials using multiple doses of cefpodoxime proxetil film-coated tablets, 4696 patients were treated with the recommended dosages of cefpodoxime (100 to 400 mg Q 12 hours). There were no deaths or permanent disabilities thought related to drug toxicity. One-hundred twenty-nine (2.7%) patients discontinued medication due to adverse events thought possibly or probably related to drug toxicity. Ninety-three (52%) of the 178 patients who discontinued therapy (whether thought related to drug therapy or not) did so because of gastrointestinal disturbances, nausea, vomiting, or diarrhea. The percentage of cefpodoxime proxetil-treated patients who discontinued study drug because of adverse events was significantly greater at a dose of 800 mg daily than at a dose of 400 mg daily or at a dose of 200 mg daily. Adverse events thought possibly or probably related to cefpodoxime in multiple-dose clinical trials (N=4696 cefpodoxime-treated patients) were:
Incidence Greater Than 1 %Diarrhea 7 % Diarrhea or loose stools were dose-related: decreasing from 10.4% of patients receiving 800 mg per day to 5.7% for those receiving 200 mg per day. Of patients with diarrhea, 10% had C. difficile organism or toxin in the stool.(See WARNINGS.)
Nausea 3.3 % Vaginal Fungal Infections 1 % Vulvovaginal Infections 1.3 % Abdominal Pain 1.2 % Headache 1 % Incidence Less Than 1 %: By body system in decreasing order
Clinical Studies
Adverse events thought possibly or probably related to cefpodoxime proxetil that occurred in less than 1% of patients (N=4696)
Body – fungal infections, abdominal distention, malaise, fatigue, asthenia, fever, chest pain, back pain, chills, generalized pain, abnormal microbiological tests, moniliasis, abscess, allergic reaction, facial edema, bacterial infections, parasitic infections, localized edema, localized pain.
Cardiovascular – congestive heart failure, migraine, palpitations, vasodilation, hematoma, hypertension, hypotension.
Digestive – vomiting, dyspepsia, dry mouth, flatulence, decreased appetite, constipation, oral moniliasis, anorexia, eructation, gastritis, mouth ulcers, gastrointestinal disorders, rectal disorders, tongue disorders, tooth disorders, increased thirst, oral lesions, tenesmus, dry throat, toothache.
Hemic and Lymphatic – anemia.
Metabolic and Nutritional – dehydration, gout, peripheral edema, weight increase.
Musculo-skeletal – myalgia.
Nervous – dizziness, insomnia, somnolence, anxiety, shakiness, nervousness, cerebral infarction, change in dreams, impaired concentration, confusion, nightmares, paresthesia, vertigo.
Respiratory – asthma, cough, epistaxis, rhinitis, wheezing, bronchitis, dyspnea, pleural effusion, pneumonia, sinusitis.
Skin – urticaria, rash, pruritus non-application site, diaphoresis, maculopapular rash, fungal dermatitis, desquamation, dry skin non-application site, hair loss, vesiculobullous rash, sunburn.
Special Senses – taste alterations, eye irritation, taste loss, tinnitus.
Urogenital – hematuria, urinary tract infections, metrorrhagia, dysuria, urinary frequency, nocturia, penile infection, proteinuria, vaginal pain.
Film-coated Tablets (Single dose)
In clinical trials using a single dose of cefpodoxime proxetil film-coated tablets, 509 patients were treated with the recommended dosage of cefpodoxime (200 mg). There were no deaths or permanent disabilities thought related to drug toxicity in these studies.
Adverse events thought possibly or probably related to cefpodoxime in single-dose clinical trials conducted in the United States were:
Incidence Greater Than 1%
Nausea 1.4 % Diarrhea 1.2 % Incidence Less Than 1 %
Central Nervous System: Dizziness, headache, syncope.
Dermatologic: Rash.
Genital: Vaginitis.
Gastrointestinal: Abdominal pain.
Psychiatric: Anxiety.
Laboratory Changes
Significant laboratory changes that have been reported in adult and pediatric patients in clinical trials of cefpodoxime proxetil, without regard to drug relationship, were:
Hepatic: Transient increases in AST (SGOT), ALT (SGPT), GGT, alkaline phosphatase, bilirubin, and LDH.
Hematologic: Eosinophilia, leukocytosis, lymphocytosis, granulocytosis, basophilia, monocytosis, thrombocytosis, decreased hemoglobin, decreased hematocrit, leukopenia, neutropenia, lymphocytopenia, thrombocytopenia, thrombocythemia, positive Coombs’ test, and prolonged PT, and PTT.
Serum Chemistry: Hyperglycemia, hypoglycemia, hypoalbuminemia, hypoproteinemia, hyperkalemia, and hyponatremia.
Renal: Increases in BUN and creatinine.
Most of these abnormalities were transient and not clinically significant.
Post-marketing Experience
The following serious adverse experiences have been reported: allergic reactions including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme and serum sickness-like reactions, pseudomembranous colitis, bloody diarrhea with abdominal pain, ulcerative colitis, rectorrhagia with hypotension, anaphylactic shock, acute liver injury, in utero exposure with miscarriage, purpuric nephritis, pulmonary infiltrate with eosinophilia, and eyelid dermatitis.
One death was attributed to pseudomembranous colitis and disseminated intravascular coagulation.
Cephalosporin Class Labeling
In addition to the adverse reactions listed above which have been observed in patients treated with cefpodoxime proxetil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics:
Adverse Reactions and Abnormal Laboratory Tests: Renal dysfunction, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, serum sickness-like reaction, hemorrhage, agranulocytosis, and pancytopenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced.(See DOSAGE AND ADMINISTRATION and OVERDOSAGE.) If seizures associated with drug therapy occur, the drug should be discontinued.
Anticonvulsant therapy can be given if clinically indicated. -
OVERDOSAGE
In acute rodent toxicity studies, a single 5 g/kg oral dose produced no adverse effects.
In the event of serious toxic reaction from overdosage, hemodialysis or peritoneal dialysis may aid in the removal of cefpodoxime from the body, particularly if renal function is compromised.
The toxic symptoms following an overdose of beta-lactam antibiotics may include nausea, vomiting, epigastric distress, and diarrhea. -
DOSAGE AND ADMINISTRATION
(See INDICATIONS AND USAGE for indicated pathogens.)
Film-coated Tablets
Cefpodoxime Proxetil Tablets, USP should be administered orally with food to enhance absorption. ( See CLINICAL PHARMACOLOGY.)
The recommended dosages, durations of treatment, and applicable patient population are as described in the following chart:Adults and Adolescents (age 12 years and older)
Type of Infection
Total Daily Dose
Dose Frequency
DurationPharyngitis and/or tonsillitis
200 mg
100 mg Q 12 hours
5 to 10 days
Acute community acquired - pneumonia
400 mg
200 mg Q 12 hours
14 days
Acute bacterial exacerbations of chronic bronchitis
400 mg
200 mg Q 12 hours
10 days
Uncomplicated gonorrhea (men and
women) and rectal gonococcal infections (women)
200 mg
single dose
Skin and skin structure
800 mg
400 mg Q 12 hours
7 to 14 days
Acute maxillary sinusitis
400 mg
200 mg Q 12 hours
10 days
Uncomplicated urinary tract infection
200 mg
100 mg Q 12 hours
7 days
Patients with Renal Dysfunction
For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
Males: Weight (kg) × (140 - age)
(mL/min) 72 × serum creatinine (mg/100 mL)
Females: 0.85 × above value
(mL/min)
Patients with Cirrhosis
Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population. -
HOW SUPPLIED
Cefpodoxime Proxetil Tablets, USP are available in the following strengths (cefpodoxime equivalent), colors, and sizes:
100 mg, (Orange colored, oval shaped, film coated tablets debossed with "A55" on one side and plain on other side)
Bottles of 20 NDC 51407-083-20
200 mg, (Orange colored, capsule shaped, film coated tablets debossed with "A57" on one side and plain on other side)
Bottles of 20 NDC 51407-084-20
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Replace cap securely after each opening. -
CLINICAL TRIALS
Cystitis
In two double-blind, 2:1 randomized, comparative trials performed in adults in the United States, cefpodoxime proxetil was compared to other beta-lactam antibiotics. In these studies, the following bacterial eradication rates were obtained at 5 to 9 days after therapy:Pathogen Cefpodoxime Comparator E. coli 200/243 (82%) 99/123 (80%) Other pathogens
K. pneumoniae
P. mirabilis
S. saprophyticus34/42 (81%) 23/28 (82%) TOTAL 234/285 (82%) 122/151 (81%) In these studies, clinical cure rates and bacterial eradication rates for cefpodoxime proxetil were comparable to the comparator agents; however, the clinical cure rates and bacteriologic eradication rates were lower than those observed with some other classes of approved agents for cystitis.
Acute Otitis Media Studies
In controlled studies of acute otitis media performed in the United States, where significant rates of beta-lactamase-producing organisms were found, cefpodoxime proxetil was compared to cefixime. In these studies, using very strict evaluability criteria and microbiologic and clinical response criteria at the 4 to 21 day post-therapy follow-up, the following presumptive bacterial eradication/clinical success outcomes (cured and improved) were obtained.Pathogen Cefpodoxime Proxetil
5 mg/kg Q 12 h x 5 dCefixime S. pneumoniae 88/122 (72%) 72/124 (58%) H. influenzae 50/76 (66%) 61/81 (75%) M. catarrhalis 22/39 (56%) 23/41 (56%) S. pyogenes 20/25 (80%) 13/23 (57%) Clinical success rate 171/254 (67%) 165/258 (64%)
Manufactured by:
Alkem Laboratories Ltd.,INDIA.
Distributed by:
Ascend Laboratories, LLC
Parsippany, NJ 07054
Revised: October, 2022PT3652
Marketed by:
GSMS, Inc.
Camarillo, CA 93012 USA
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CEFPODOXIME PROXETIL
cefpodoxime proxetil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-084(NDC:67877-879) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFPODOXIME PROXETIL (UNII: 2TB00A1Z7N) (CEFPODOXIME - UNII:7R4F94TVGY) CEFPODOXIME 200 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape CAPSULE Size 16mm Flavor Imprint Code A57 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-084-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210568 05/18/2022 CEFPODOXIME PROXETIL
cefpodoxime proxetil tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-083(NDC:67877-878) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CEFPODOXIME PROXETIL (UNII: 2TB00A1Z7N) (CEFPODOXIME - UNII:7R4F94TVGY) CEFPODOXIME 100 mg Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSES (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color orange Score no score Shape OVAL Size 12mm Flavor Imprint Code A55 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-083-20 20 in 1 BOTTLE; Type 0: Not a Combination Product 04/03/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210568 05/18/2022 Labeler - Golden State Medical Supply, Inc. (603184490) Establishment Name Address ID/FEI Business Operations Golden State Medical Supply, Inc. 603184490 relabel(51407-083, 51407-084) , repack(51407-083, 51407-084)




