Label: STENDRA- avanafil tablet
-
NDC Code(s):
72384-751-30,
72384-752-01,
72384-752-30,
72384-752-99, view more72384-753-01, 72384-753-30, 72384-753-99
- Packager: Metuchen Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use STENDRA safely and effectively. See full prescribing information for STENDRA.
STENDRA® (avanafil) tablets, for oral use
Initial U.S. Approval: 2012
INDICATIONS AND USAGE
STENDRA is a phosphodiesterase 5 (PDE5) inhibitor indicated for the treatment of erectile dysfunction (1)
DOSAGE AND ADMINISTRATION
- The starting dose is 100 mg taken as early as approximately 15 minutes before sexual activity, on an as needed basis (2.1)
- Take STENDRA no more than once a day (2.1).
- Based on efficacy and/or tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity, or decreased to 50 mg taken approximately 30 minutes before sexual activity. Use the lowest dose that provides benefit (2.1).
- STENDRA may be taken with or without food (2.2)
- Do not use STENDRA with strong CYP3A4 inhibitors (2.3)
- If taking a moderate CYP3A4 inhibitor, the dose should be no more than 50 mg in a 24-hour period (2.3).
- In patients on stable alpha-blocker therapy, the recommended starting dose of STENDRA is 50 mg (2.3).
DOSAGE FORMS AND STRENGTHS
Tablets: 50 mg, 100 mg, 200 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Patients should not use STENDRA if sexual activity is inadvisable due to cardiovascular status or any other reason (5.1)
- Use of STENDRA with alpha-blockers, other antihypertensives, or substantial amounts of alcohol (greater than 3 units) may lead to hypotension (2.3, 5.6, 5.7)
- Patients should seek emergency treatment if an erection lasts greater than 4 hours (5.3)
- Patients should stop STENDRA and seek medical care if a sudden loss of vision occurs in one or both eyes, which could be a sign of Non Arteritic Ischemic Optic Neuropathy (NAION). STENDRA should be used with caution, and only when the anticipated benefits outweigh the risks, in patients with a history of NAION. Patients with a “crowded” optic disc may also be at an increased risk of NAION (5.4, 6.2)
- Patients should stop taking STENDRA and seek prompt medical attention in the event of sudden decrease or loss of hearing (5.5)
ADVERSE REACTIONS
Most common adverse reactions (greater than or equal to 2%) include headache, flushing, nasal congestion, nasopharyngitis, and back pain (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact 844.458.4887 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 10/2022
- The starting dose is 100 mg taken as early as approximately 15 minutes before sexual activity, on an as needed basis (2.1)
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Erectile Dysfunction
2.2 Use with Food
2.3 Concomitant Medications
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Nitrates
4.2 Hypersensitivity Reactions
4.3 Concomitant Guanylate Cyclase (GC) Stimulators
5 WARNINGS AND PRECAUTIONS
5.1 Cardiovascular Risks
5.2 Concomitant Use of CYP3A4 Inhibitors
5.3 Prolonged Erection
5.4 Effects on Eye
5.5 Sudden Hearing Loss
5.6 Alpha-Blockers and Other Antihypertensives
5.7 Alcohol
5.8 Combination with Other PDE5 Inhibitors or Erectile Dysfunction Therapies
5.9 Effects on Bleeding
5.10 Counseling Patients about Sexually Transmitted Diseases
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for Pharmacodynamic Interactions with STENDRA
7.2 Potential for Other Drugs to Affect STENDRA
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Nitrates
17.2 Cardiovascular Considerations
17.3 Concomitant Use with Drugs Which Lower Blood Pressure
17.4 Potential for Drug Interactions
17.5 Priapism
17.6 Vision
17.7 Sudden Hearing Loss
17.8 Alcohol
17.9 Sexually Transmitted Disease
17.10 Recommended Administration
17.11 Guanylate Cyclase (GC) Stimulators
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Erectile Dysfunction
The recommended starting dose is 100 mg. STENDRA should be taken orally as needed as early as approximately 15 minutes before sexual activity.
Based on individual efficacy and tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity, or decreased to 50 mg taken approximately 30 minutes before sexual activity. The lowest dose that provides benefit should be used.
The maximum recommended dosing frequency is once per day. Sexual stimulation is required for a response to treatment.
2.3 Concomitant Medications
Nitrates
Concomitant use of nitrates in any form is contraindicated [see Contraindications (4.1)].
Alpha-Blockers
If STENDRA is co-administered with an alpha-blocker, patients should be stable on alpha-blocker therapy prior to initiating treatment with STENDRA, and STENDRA should be initiated at the 50 mg dose [see Warnings and Precautions (5.6), Drug Interactions (7.1) and Clinical Pharmacology (12.2)].
CYP3A4 Inhibitors
- For patients taking concomitant strong CYP3A4 inhibitors (including ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin), do not use STENDRA [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
- For patients taking concomitant moderate CYP3A4 inhibitors (including erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil), the maximum recommended dose of STENDRA is 50 mg, not to exceed once every 24 hours [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Nitrates
Administration of STENDRA with any form of organic nitrates, either regularly and/or intermittently, is contraindicated. Consistent with its known effects on the nitric oxide/cyclic guanosine monophosphate (cGMP) pathway, STENDRA has been shown to potentiate the hypotensive effects of nitrates.
In a patient who has taken STENDRA, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of STENDRA before nitrate administration is considered. In such circumstances, nitrates should only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications (4.1), Dosage and Administration (2.3), and Clinical Pharmacology (12.2)].
-
5 WARNINGS AND PRECAUTIONS
Evaluation of erectile dysfunction (ED) should include an appropriate medical assessment to identify potential underlying causes, as well as treatment options.
Before prescribing STENDRA, it is important to note the following:
5.1 Cardiovascular Risks
There is a potential for cardiac risk during sexual activity in patients with pre-existing cardiovascular disease. Therefore, treatments for ED, including STENDRA, should not be used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status.
Patients with left ventricular outflow obstruction (e.g., aortic stenosis, idiopathic hypertrophic subaortic stenosis) and those with severely impaired autonomic control of blood pressure can be particularly sensitive to the actions of vasodilators, including STENDRA.
The following groups of patients were not included in clinical safety and efficacy trials for STENDRA, and therefore until further information is available, STENDRA is not recommended for the following groups:
- Patients who have suffered a myocardial infarction, stroke, life-threatening arrhythmia, or coronary revascularization within the last 6 months;
- Patients with resting hypotension (blood pressure less than 90/50 mmHg) or hypertension (blood pressure greater than 170/100 mmHg);
- Patients with unstable angina, angina with sexual intercourse, or New York Heart Association Class 2 or greater congestive heart failure.
As with other PDE5 inhibitors STENDRA has systemic vasodilatory properties and may augment the blood pressure-lowering effect of other anti-hypertensive medications. STENDRA 200 mg resulted in transient decreases in sitting blood pressure in healthy volunteers of 8.0 mmHg systolic and 3.3 mmHg diastolic [see Clinical Pharmacology (12.2)], with the maximum decrease observed at 1 hour after dosing. While this normally would be expected to be of little consequence in most patients, prior to prescribing STENDRA, physicians should carefully consider whether patients with underlying cardiovascular disease could be affected adversely by such vasodilatory effects, especially in combination with sexual activity.
5.2 Concomitant Use of CYP3A4 Inhibitors
STENDRA metabolism is principally mediated by the CYP450 isoform 3A4 (CYP3A4). Inhibitors of CYP3A4 may reduce STENDRA clearance and increase plasma concentrations of avanafil.
For patients taking concomitant strong CYP3A4 inhibitors (including ketoconazole, ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir and telithromycin), do not use STENDRA [see Drug Interactions (7.2)].
For patients taking concomitant moderate CYP3A4 inhibitors (including erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil), the maximum recommended dose of STENDRA is 50 mg, not to exceed once every 24 hours [see Drug Interactions (7.2)].
5.3 Prolonged Erection
Prolonged erection greater than 4 hours and priapism (painful erections greater than 6 hours in duration) have been reported with other PDE5 inhibitors. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If not treated immediately, penile tissue damage and permanent loss of potency could result.
STENDRA should be used with caution in patients with anatomical deformation of the penis (such as angulation, cavernosal fibrosis, or Peyronie’s disease), or in patients who have conditions which may predispose them to priapism (such as sickle cell anemia, multiple myeloma, or leukemia).
5.4 Effects on Eye
Physicians should advise patients to stop use of all PDE5 inhibitors, including STENDRA and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a rare condition and a cause of decreased vision including permanent loss of vision that has been reported rarely postmarketing in temporal association with the use of all PDE5 inhibitors. Based on published literature, the annual incidence of NAION is 2.5-11.8 cases per 100,000 in males aged ≥ 50.
An observational case-crossover study evaluated the risk of NAION when PDE5 inhibitor use, as a class, occurred immediately before NAION onset (within 5 half-lives), compared to PDE5 inhibitor use in a prior time period. The results suggest an approximate 2-fold increase in the risk of NAION, with a risk estimate of 2.15 (95% CI 1.06, 4.34). A similar study reported a consistent result, with a risk estimate of 2.27 (95% CI 0.99, 5.20). Other risk factors for NAION, such as the presence of “crowded” optic disc, may have contributed to the occurrence of NAION in these studies.
Neither the rare postmarketing reports, nor the association of PDE5 inhibitor use and NAION in the observational studies, substantiate a causal relationship between PDE5 inhibitor use and NAION [see Adverse Reactions (6.2)].
Physicians should consider whether their patients with underlying NAION risk factors could be adversely affected by use of PDE5 inhibitors. Individuals who have already experienced NAION are at increased risk of NAION recurrence. Therefore, PDE5 inhibitors, including STENDRA should be used with caution in these patients and only when the anticipated benefits outweigh the risks. Individuals with “crowded” optic disc are also considered at greater risk for NAION compared to the general population; however, evidence is insufficient to support screening of prospective users of PDE5 inhibitors, including STENDRA, for this uncommon condition.
Patients with known hereditary degenerative retinal disorders, including retinitis pigmentosa, were not included in the clinical trials for STENDRA, and therefore, until further information is available, STENDRA is not recommended for use in these patients.
5.5 Sudden Hearing Loss
Use of PDE5 inhibitors has been associated with sudden decrease or loss of hearing, which may be accompanied by tinnitus or dizziness. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions (6)]. Patients experiencing these symptoms should be advised to stop taking STENDRA and seek prompt medical attention.
5.6 Alpha-Blockers and Other Antihypertensives
Physicians should discuss with patients the potential for STENDRA to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications [see Drug Interactions (7.1) and Clinical Pharmacology (12.2)].
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. Phosphodiesterase type 5 inhibitors, including STENDRA, and alpha-adrenergic blocking agents are both vasodilators with blood pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (e.g., dizziness, lightheadedness, fainting).
Consideration should be given to the following:
- Patients should be stable on alpha-blocker therapy prior to initiating treatment with a PDE5 inhibitor. Patients who demonstrate hemodynamic instability on alpha-blocker therapy alone are at increased risk of symptomatic hypotension with concomitant use of PDE5 inhibitors.
- In those patients who are stable on alpha-blocker therapy, PDE5 inhibitors should be initiated at the lowest dose (STENDRA 50 mg).
- In those patients already taking an optimized dose of a PDE5 inhibitor, alpha-blocker therapy should be initiated at the lowest dose. Stepwise increase in alpha-blocker dose may be associated with further lowering of blood pressure when taking a PDE5 inhibitor.
Safety of combined use of PDE5 inhibitors and alpha-blockers may be affected by other variables, including intravascular volume depletion and other anti-hypertensive drugs [see Dosage and Administration (2) and Drug Interactions (7.1)].
5.7 Alcohol
Patients should be made aware that both alcohol and PDE5 inhibitors including STENDRA act as vasodilators. When vasodilators are taken in combination, blood-pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., greater than 3 units) in combination with STENDRA may increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Drug Interactions (7.1) and Clinical Pharmacology (12.2)].
5.8 Combination with Other PDE5 Inhibitors or Erectile Dysfunction Therapies
The safety and efficacy of combinations of STENDRA with other treatments for ED has not been studied. Therefore, the use of such combinations is not recommended.
5.9 Effects on Bleeding
The safety of STENDRA is unknown in patients with bleeding disorders and patients with active peptic ulceration. In vitro studies with human platelets indicate that STENDRA potentiates the anti-aggregatory effect of sodium nitroprusside (a nitric oxide [NO] donor).
5.10 Counseling Patients about Sexually Transmitted Diseases
The use of STENDRA offers no protection against sexually transmitted diseases. Counseling patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV), should be considered.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
STENDRA was administered to 2215 men during clinical trials. In trials of STENDRA for use as needed, a total of 493 patients were exposed for greater than or equal to 6 months, and 153 patients were treated for greater than or equal to 12 months.
In three randomized, double-blind, placebo-controlled trials lasting up to 3 months in duration, the mean age of patients was 56.4 years (range from 23 to 88 years). 83.9% of patients were White, 13.8% were Black, 1.4% Asian, and < 1% Hispanic. 41.1% were current or previous smokers. 30.6% had diabetes mellitus.
The discontinuation rate due to adverse reactions for patients treated with STENDRA 50 mg, 100 mg, or 200 mg was 1.4%, 2.0%, and 2.0%, respectively, compared to 1.7% for placebo-treated patients.
Table 1 presents the adverse reactions reported when STENDRA was taken as recommended (on an as-needed basis) from these 3 clinical trials.
Table 1: Adverse Reactions Reported by Greater Than or Equal to 2% of Patients Treated with STENDRA From 3 Placebo-Controlled Clinical Trials Lasting 3 Months for STENDRA Use as Needed Adverse Reaction Placebo
(N = 349)STENDRA
50 mg
(N = 217)STENDRA
100 mg
(N = 349)STENDRA
200 mg
(N = 352)Headache 1.7% 5.1% 6.9% 10.5% Flushing 0.0% 3.2% 4.3% 4.0% Nasal congestion 1.1% 1.8% 2.9% 2.0% Nasopharyngitis 2.9% 0.9% 2.6% 3.4% Back pain 1.1% 3.2% 2.0% 1.1% Adverse reactions reported by greater than or equal to 1%, but less than 2% of patients in any STENDRA dose group, and greater than placebo included: upper respiratory infection (URI), bronchitis, influenza, sinusitis, sinus congestion, hypertension, dyspepsia, nausea, constipation, and rash.
In an open-label, long-term extension study of two of these randomized, double-blind, placebo-controlled trials, the total duration of treatment was up to 52 weeks. Among the 712 patients who participated in this open-label extension study, the mean age of the population was 56.4 years (range from 23 to 88 years). The discontinuation rate due to adverse reactions for patients treated with STENDRA (50 mg, 100 mg, or 200 mg) was 2.8%.
In this extension trial, all eligible patients were initially assigned to STENDRA 100 mg. At any point during the trial, patients could request to have their dose of STENDRA increased to 200 mg or decreased to 50 mg based on their individual response to treatment. In total, 536 (approximately 75%) patients increased their dose to 200 mg and 5 (less than 1%) patients reduced their dose to 50 mg.
Table 2 presents the adverse reactions reported when STENDRA was taken as recommended (on an as-needed basis) in this open-label extension trial.
Table 2: Adverse Reactions Reported by Greater Than or Equal to 2% of Patients Treated With STENDRA in an Open-Label Extension Trial Adverse Reaction STENDRA
(N = 711)Headache 5.6% Flushing 3.5% Nasopharyngitis 3.4% Nasal congestion 2.1% Adverse reactions reported by greater than or equal to 1%, but less than 2% of patients in the open-label extension study included: upper respiratory infection (URI), influenza, sinusitis, bronchitis, dizziness, back pain, arthralgia, hypertension, and diarrhea.
The following events occurred in less than 1% of patients in the three placebo-controlled 3-month clinical trials and/or the open-label, long-term extension study lasting 12 months. A causal relationship to STENDRA is uncertain. Excluded from this list are those events that were minor, those with no plausible relation to drug use and reports too imprecise to be meaningful.
Body as a whole — edema peripheral, fatigue
Cardiovascular — angina, unstable angina, deep vein thrombosis, palpitations
Digestive — gastritis, gastroesophageal reflux disease, hypoglycemia, blood glucose increased, alanine aminotransferase increased, oropharyngeal pain, stomach discomfort, vomiting
Musculoskeletal — muscle spasms, musculoskeletal pain, myalgia, pain in extremity
Nervous — depression, insomnia, somnolence, vertigo
Respiratory — cough, dyspnea exertional, epistaxis, wheezing
Skin and Appendages — pruritus
Urogenital — balanitis, erection increased, hematuria, nephrolithiasis, pollakiuria, urinary tract infection
In an additional, randomized, double-blind, placebo-controlled study lasting up to 3 months in 298 men who had undergone bilateral nerve-sparing radical prostatectomy for prostate cancer, the mean age of patients was 58.4 years (range 40 – 70). Table 3 presents the adverse reactions reported in this additional study.
Table 3: Adverse Reactions Reported by Greater than or Equal to 2% of Patients Treated with STENDRA in a Placebo-Controlled Clinical Trial Lasting 3 Months in Patients Who Underwent Bilateral Nerve-Sparing Radical Prostatectomy (Study 3) Adverse Reaction Placebo
(N = 100)STENDRA
100 mg
(N = 99)STENDRA
200 mg
(N = 99)Headache 1.0% 8.1% 12.1% Flushing 0.0% 5.1% 10.1% Nasopharyngitis 0.0% 3.0% 5.1% Upper respiratory infection 0.0% 2.0% 3.0% Nasal congestion 1.0% 3.0% 1.0% Back pain 1.0% 3.0% 2.0% Electrocardiogram abnormal 0.0% 1.0% 3.0% Dizziness 0.0% 1.0% 2.0% A randomized, double-blind, placebo-controlled 2 months study was conducted in 435 subjects with a mean age of 58.2 years (range 24 to 86 years) to determine the time to onset of effect of STENDRA, defined as the time to the first occurrence of an erection sufficient for sexual intercourse. Table 4 presents the adverse reactions occurring in ≥ 2% of subjects treated with STENDRA.
Table 4: Adverse Reactions Reported by ≥ 2% of Patients Treated with STENDRA in a Placebo-Controlled Clinical Trial Lasting 2 Months to Determine the Time to Onset of Effect (Study 4) Adverse Reaction Placebo
(N = 143)STENDRA
100 mg
(N = 146)STENDRA
200 mg
(N = 146)Headache 0.7% 1.4% 8.9% Nasal congestion 0.0% 0.7% 4.1% Gastroenteritis viral 0.0% 0.0% 2.1% Across all trials with any STENDRA dose, 1 subject reported a change in color vision.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of STENDRA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion either due to their seriousness, reporting frequency, lack of clear alternative causation, or a combination of these factors.
Cardiovascular and cerebrovascular:
Serious cardiovascular and cerebrovascular events, including myocardial infarction, sudden cardiac death, cerebrovascular accident, and subarachnoid hemorrhage, have been reported post-marketing in temporal association with the use of STENDRA. All of these patients had preexisting cardiovascular risk factors. The occurrence of sexual activity was not stated in the majority of reports, although events were reported to occur both with and without sexual activity. It is not possible to determine whether these events are related directly to STENDRA, to sexual activity, to the patient’s underlying cardiovascular disease, to a combination of these factors, or to other factors [see Warnings and Precautions (5.1) and Patient Counseling Information (17.2)].
Nervous: transient global amnesia
Special senses:
Ophthalmologic: vitreous detachment, visual impairment
Non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision, has been reported rarely post-marketing in temporal association with the use of phosphodiesterase type 5 (PDE5) inhibitors, such as STENDRA. Most, but not all, of these patients had underlying anatomic or vascular risk factors for developing NAION, including but not necessarily limited to: low cup to disc ratio (“crowded disc”), age over 50, diabetes, hypertension, coronary artery disease, hyperlipidemia and smoking [see Warnings and Precautions (5.4)].
Otologic: Cases of sudden decrease or loss of hearing have been reported postmarketing in temporal association with the use of PDE5 inhibitors, such as STENDRA. In some of the cases, medical conditions and other factors were reported that may have also played a role in the otologic adverse events. In many cases, medical follow-up information was limited. It is not possible to determine whether these reported events are related directly to the use of PDE5 inhibitors such as STENDRA, to the patient’s underlying risk factors for hearing loss, a combination of these factors, or to other factors [see Warnings and Precautions (5.5) and Patient Counseling Information (17.6)].
-
7 DRUG INTERACTIONS
7.1 Potential for Pharmacodynamic Interactions with STENDRA
Nitrates
Administration of STENDRA to patients who are using any form of organic nitrate, is contraindicated. In a clinical pharmacology trial, STENDRA was shown to potentiate the hypotensive effect of nitrates. In a patient who has taken STENDRA, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of STENDRA before nitrate administration is considered. In such circumstances, nitrates should only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications (4.1), Dosage and Administration (2.3), and Clinical Pharmacology (12.2)].
Alpha-Blockers
Caution is advised when PDE5 inhibitors are co-administered with alpha-blockers. PDE5 inhibitors, including STENDRA, and alpha-adrenergic blocking agents are both vasodilators with blood pressure-lowering effects. When vasodilators are used in combination, an additive effect on blood pressure may be anticipated. In some patients, concomitant use of these two drug classes can lower blood pressure significantly leading to symptomatic hypotension (e.g., dizziness, lightheadedness, fainting) [see Warnings and Precautions (5.6), Dosage and Administration (2.3), and Clinical Pharmacology (12.2)].
Antihypertensives
PDE5 inhibitors, including STENDRA, are mild systemic vasodilators. A clinical pharmacology trial was conducted to assess the effect of STENDRA on the potentiation of the blood pressure-lowering effects of selected antihypertensive medications (amlodipine and enalapril). Additional reductions in blood pressure of 3 to 5 mmHg occurred following co-administration of a single 200 mg dose of STENDRA with these agents compared with placebo [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.2)].
Alcohol
Both alcohol and PDE5 inhibitors, including STENDRA, act as vasodilators. When vasodilators are taken in combination, blood pressure-lowering effects of each individual compound may be increased. Substantial consumption of alcohol (e.g., greater than 3 units) in combination with STENDRA can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Clinical Pharmacology (12.2)].
7.2 Potential for Other Drugs to Affect STENDRA
STENDRA is a substrate of and predominantly metabolized by CYP3A4. Studies have shown that drugs that inhibit CYP3A4 can increase avanafil exposure.
Strong CYP3A4 Inhibitors
Ketoconazole (400 mg daily), a selective and strong inhibitor of CYP3A4, increased STENDRA 50 mg single-dose systemic exposure (AUC) and maximum concentration (Cmax) equal to 13-fold and 3-fold, respectively, and prolonged the half-life of avanafil to approximately 9 hours. Other potent inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, nefazadone, ritonavir, saquinavir, nelfinavir, indinavir, atanazavir and telithromycin) would be expected to have similar effects. Do not use STENDRA in patients taking strong CYP3A4 inhibitors [see Warnings and Precautions (5.2) and Dosage and Administration (2.3)].
HIV Protease inhibitor — Ritonavir (600 mg twice daily), a strong CYP3A4 inhibitor, which also inhibits CYP2C9, increased STENDRA 50 mg single-dose Cmax and AUC equal to approximately 2-fold and 13-fold, and prolonged the half-life of avanafil to approximately 9 hours in healthy volunteers. Do not use STENDRA in patients taking ritonavir.
Moderate CYP 3A4 Inhibitors
Erythromycin (500 mg twice daily) increased STENDRA 200 mg single-dose Cmax and AUC equal to approximately 2-fold and 3-fold, respectively, and prolonged the half-life of avanafil to approximately 8 hours in healthy volunteers. Moderate CYP3A4 inhibitors (e.g., erythromycin, amprenavir, aprepitant, diltiazem, fluconazole, fosamprenavir, and verapamil) would be expected to have similar effects. Consequently, the maximum recommended dose of STENDRA is 50 mg, not to exceed once every 24 hours for patients taking concomitant moderate CYP3A4 inhibitors [see Warnings and Precautions (5.2) and Drug Interactions (7.2)].
Although specific interactions have not been studied, other CYP3A4 inhibitors, including grapefruit juice are likely to increase avanafil exposure.
Weak CYP3A4 Inhibitors
No in vivo drug-drug interaction studies with weak CYP3A4 inhibitors were conducted.
CYP3A4 Substrate
When administered with STENDRA 200 mg, amlodipine (5 mg daily) increased the Cmax and AUC of avanafil by approximately 22% and 70%, respectively. The half-life of STENDRA was prolonged to approximately 10 hrs. The Cmax and AUC of amlodipine decreased by approximately 9% and 4%, respectively [see Dosage and Administration (2.3)].
Cytochrome P450 Inducers
The potential effect of CYP inducers on the pharmacokinetics of avanafil was not evaluated. The concomitant use of STENDRA and CYP inducers is not recommended.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
STENDRA is not indicated for use in females.
There are no data with the use of STENDRA in pregnant women to inform any drug-associated risks for adverse developmental outcomes. In animal reproduction studies conducted in pregnant rats and rabbits, no adverse developmental outcomes were observed with oral administration of avanafil during organogenesis at exposures for total avanafil at approximately 8 and 6 times, respectively, the Maximum Recommended Human Dose (MRHD) of 200 mg based on AUC (see Data).
Data
Animal Data
In pregnant rats administered orally at 100, 300, or 1000 mg/kg/day from gestation days 6 to 17, no evidence of teratogenicity, embryotoxicity, or fetotoxicity was observed at up to 300 mg/kg/day. This dose is equivalent to exposures of approximately 8 times the exposure at the Maximum Recommended Human Dose (MRHD) of 200 mg based on AUCs for total avanafil. At the maternally toxic dose (1000 mg/kg/day), decreased fetal body weight occurred with no signs of teratogenicity. In pregnant rabbits administered orally at 30, 60, 120, or 240 mg/kg/day from gestation days 6 to 18, no teratogenicity was observed at exposures up to approximately 6 times the human exposure at the MRHD based on AUCs for total avanafil.
In a pre- and post-natal development study in rats given orally at 100, 300, or 600 mg/kg/day on gestation days 6 through lactation day 20, offspring growth and maturation were reduced when maternal rats were given avanafil doses greater than or equal to 300 mg/kg/day, resulting in exposures greater than or equal to 17 times the human exposure to total avanafil at the MRHD. There was no effect on reproductive performance of the maternal rats or offspring, or on the behavior of the offspring at up to the highest dose tested. The no observed adverse effect level (NOAEL) for developmental toxicity (100 mg/kg/day) was observed at exposures to total avanafil approximately 2-fold greater than the systemic exposure in humans at the MRHD.
8.2 Lactation
Risk Summary
STENDRA is not indicated for use in females.
There is no information on the presence of avanafil and/or its metabolites in human or animal milk, the effects on the breastfed child, or the effects on milk production.
8.3 Females and Males of Reproductive Potential
Infertility
There have been no studies evaluating the effect of STENDRA on fertility in men [see Clinical Pharmacology (12.2)]
Based on studies in animals, decreased fertility, abnormal sperm motility and morphology, and altered estrous cycles were observed in rats. The abnormal sperm findings were reversible at the end of a 9-week drug-free period [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
STENDRA is not indicated for use in pediatric patients. Safety and efficacy in patients below the age of 18 years has not been established.
8.5 Geriatric Use
Of the total number of subjects in clinical studies of avanafil, approximately 23% were 65 and over. No overall differences in efficacy and safety were observed between subjects over 65 years of age compared to younger subjects; therefore, no dose adjustment is warranted based on age alone. However, a greater sensitivity to medication in some older individuals should be considered [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
In a clinical pharmacology trial using single 200 mg doses of STENDRA, avanafil exposure (AUC or Cmax) in normal subjects was comparable to patients with mild (creatinine clearance greater than or equal to 60 to less than 90 mL/min) or moderate (creatinine clearance greater than or equal to 30 to less than 60 mL/min) renal impairment. No dose adjustment is necessary for patients with mild to moderate renal impairment (creatinine clearance greater than or equal to 30 to less than 90 mL/min). The pharmacokinetics of avanafil in patients with severe renal disease or on renal dialysis has not been studied; do not use STENDRA in such patients [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
In a clinical pharmacology trial, avanafil AUC and Cmax in patients with mild hepatic impairment (Child-Pugh Class A) was comparable to that in healthy subjects when a dose of 200 mg was administered. Avanafil Cmax was approximately 51% lower and AUC was 11% higher in patients with moderate hepatic impairment (Child Pugh Class B) compared to subjects with normal hepatic function. No dose adjustment is necessary for patients with mild to moderate hepatic impairment (Child Pugh Class A or B). The pharmacokinetics of avanafil in patients with severe hepatic disease has not been studied; do not use STENDRA in such patients [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Single doses up to 800 mg have been given to healthy subjects, and multiple doses up to 300 mg have been given to patients. In cases of overdose, standard supportive measures should be adopted as required. Renal dialysis is not expected to accelerate clearance because avanafil is highly bound to plasma proteins and is not significantly eliminated in the urine.
-
11 DESCRIPTION
STENDRA (avanafil) is a selective inhibitor of cGMP-specific PDE5.
Avanafil is designated chemically as (S)-4-[(3-Chloro-4-methoxybenzyl)amino]-2-[2-(hydroxymethyl)-1-pyrrolidinyl]-N-(2-pyrimidinylmethyl)-5-pyrimidinecarboxamide and has the following structural formula:
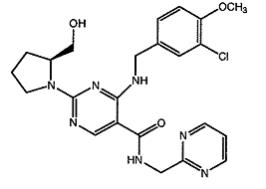
Avanafil occurs as white crystalline powder, molecular formula C23H26ClN7O3 and molecular weight of 483.95 and is slightly soluble in ethanol, practically insoluble in water, soluble in 0.1 mol/L hydrochloric acid. STENDRA, for oral administration, is supplied as oval, pale yellow tablets containing 50 mg, 100 mg, or 200 mg avanafil debossed with dosage strengths. In addition to the active ingredient, avanafil, each tablet contains the following inactive ingredients: mannitol, fumaric acid, hydroxypropylcellulose, low substituted hydroxypropylcellulose, calcium carbonate, magnesium stearate, and ferric oxide yellow.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The physiologic mechanism of erection of the penis involves release of nitric oxide (NO) in the corpus cavernosum during sexual stimulation. NO then activates the enzyme guanylate cyclase, which results in increased levels of cGMP, producing smooth muscle relaxation in the corpus cavernosum and allowing inflow of blood. Avanafil has no direct relaxant effect on isolated human corpus cavernosum, but enhances the effect of NO by inhibiting PDE5, which is responsible for degradation of cGMP in the corpus cavernosum. Because sexual stimulation is required to initiate the local release of nitric oxide, the inhibition of PDE5 has no effect in the absence of sexual stimulation.
Studies in vitro have shown that avanafil is selective for PDE5. Its effect is more potent on PDE5 than on other known phosphodiesterases (greater than 100-fold for PDE6; greater than 1,000-fold for PDE4, PDE8 and PDE10; greater than 5,000-fold for PDE2 and PDE7; greater than 10,000-fold for PDE1, PDE3, PDE9, and PDE11). Avanafil is greater than 100-fold more potent for PDE5 than PDE6, which is found in the retina and is responsible for phototransduction. In addition to human corpus cavernosum smooth muscle, PDE5 is also found in other tissues including platelets, vascular and visceral smooth muscle, and skeletal muscle, brain, heart, liver, kidney, lung, pancreas, prostate, bladder, testis, and seminal vesicle. The inhibition of PDE5 in these tissues by avanafil may be the basis for the enhanced platelet anti-aggregatory activity of NO observed in vitro and peripheral vasodilatation in vivo.
12.2 Pharmacodynamics
Effects of STENDRA on Erectile Response
In a single-blind, placebo-controlled, single-dose trial of 82 patients with either organic and/or psychogenic ED, visual sexual stimulation resulted in improved erections after STENDRA administration compared to placebo, as assessed by an objective measurement of hardness and duration of erections (RigiScan®). Efficacy was assessed by RigiScan at discrete time intervals ranging from 20 – 40 minutes after dosing to 100 – 120 minutes after dosing.
Effects of STENDRA on Blood Pressure
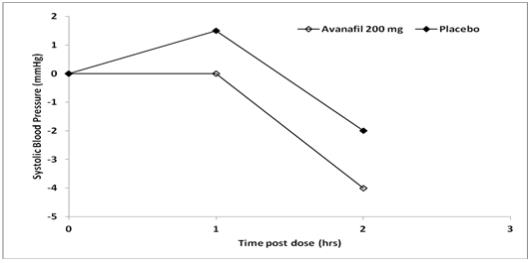
Single oral doses of STENDRA (200 mg) administered to healthy male volunteers resulted in mean changes from baseline in systolic/diastolic blood pressure of -5.3/-3.7 mmHg at 1 hour after dosing, compared to mean changes from baseline in the placebo group of 2.7/-0.4 mmHg. The reductions in systolic/diastolic blood pressure at 1 hour after dosing of STENDRA 200 mg compared to placebo were 8.0/3.3 mmHg.
Figure 1: Median Change from Baseline in Sitting Systolic Blood Pressure, Healthy Volunteers Day 4
Effects on Cardiac Electrophysiology
The effect of single 100 or 800 mg doses of STENDRA on the QT interval were evaluated in a randomized, double-blind, placebo, and active (moxifloxacin) –controlled crossover study in 52 healthy male subjects aged 18 to 45 years. There were no significant effects of the 100 mg dose. The mean QTc (Fridericia QT correction) for avanafil 800 mg, relative to placebo was 9.4 milliseconds (two-sided 90% CI=7.2, 11.6). An 800 mg dose of STENDRA (4 times the highest recommended dose) was chosen because this dose yields exposures greater than those observed upon co-administration of avanafil with strong CYP3A4 inhibitors. A double-blind, randomized, placebo- and active-controlled (moxifloxacin), thorough QT/QTc trial of STENDRA (100 and 800 mg) in healthy male subjects demonstrated that STENDRA did not cause any significant changes in QTc interval or ventricular repolarization.
Effects of STENDRA on Blood Pressure When Administered with Nitrates
In a clinical pharmacology trial, a single dose of STENDRA 200 mg was shown to potentiate the hypotensive effect of nitrates. The use of STENDRA in patients taking any form of nitrates is contraindicated [see Contraindications (4.1)].
A trial was conducted to assess the degree of interaction between nitroglycerin and STENDRA, should nitroglycerin be required in an emergency situation after STENDRA was taken. This was a single-center, double-blind, randomized, 3-way crossover trial of healthy males from 30 to 60 years of age. Subjects were divided among 5 trial groups with the trial group being determined by the time interval between treatment with trial drug and glyceryl trinitrate administration. Subjects were assigned to trial groups sequentially and hemodynamic results from the previous group were reviewed for serious adverse events (SAEs) before the next group received treatment. Each subject was dosed with all 3 study drugs (STENDRA 200 mg, sildenafil citrate 100 mg, and placebo) in random order. Subjects were administered a single dose of 0.4 mg sublingual nitroglycerin (NTG) at pre-specified time points, following their dose of trial drug (0.5, 1, 4, 8 or 12 hours). Overall, 14 (15%) subjects treated with placebo and 28 (28%) subjects treated with avanafil, had clinically significant decreases in standing SBP, defined as greater than or equal to 30 mmHg decrease in SBP, after glyceryl trinitrate administration. Mean maximum decreases are shown in Table 5.
Table 5: Mean Maximum Decreases from Baseline in Sitting and Standing Systolic Blood Pressure/Diastolic Blood Pressure (mmHg) following Placebo or 200 mg STENDRA with 0.4 mg sublingual nitroglycerin Placebo with nitroglycerin
Sitting
Standing13.4/11.8
21.1/16.5STENDRA with nitroglycerin
Sitting
Standing
21.6/18.2
28.0/23.5Like other PDE5 inhibitors, STENDRA administration with nitrates is contraindicated. In a patient who has taken STENDRA, where nitrate administration is deemed medically necessary in a life threatening situation, at least 12 hours should elapse after the last dose of STENDRA before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring [see Contraindications (4.1)].
Effects of STENDRA on Blood Pressure When Administered with Alpha-Blockers
A single-center, randomized, double-blinded, placebo-controlled, two-period crossover trial was conducted to investigate the potential interaction of STENDRA with alpha-blocker agents in healthy male subjects which consisted of two cohorts:
Cohort A (N=24): Subjects received oral doses of doxazosin once daily in the morning at 1 mg for 1 day (Day 1), 2 mg for 2 days (Days 2 – 3), 4 mg for 4 days (Days 4 – 7), and 8 mg for 11 days (Days 8 – 18). On Days 15 and 18, the subjects also received a single oral dose of either 200 mg STENDRA or placebo, according to the treatment randomization code. The STENDRA or placebo doses were administered 1.3 hours after the doxazosin administration on Days 15 and 18. The co-administration was designed so that doxazosin (Tmax ~2 hours) and STENDRA (Tmax ~0.7 hours) would reach their peak plasma concentrations at the same time.
Cohort B (N=24): Subjects received 0.4 mg daily oral doses of tamsulosin in the morning for 11 consecutive days (Days 1 – 11). On Days 8 and 11, the subjects also received a single oral dose of either 200 mg STENDRA or placebo, according to the treatment randomization code. The STENDRA or placebo doses were administered 3.3 hours after the tamsulosin administration on Days 8 and 11. The co-administration was designed so that tamsulosin (Tmax ~4 hours) and STENDRA (Tmax ~0.7 hours) would reach their peak plasma concentrations at the same time.
Supine and sitting BP and pulse rate measurements were recorded before and after STENDRA or placebo dosing.
A total of seven subjects in Cohort A (doxazosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Three subjects experienced standing SBP values less than 85 mmHg. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following STENDRA. Two subjects experienced standing DBP values less than 45 mmHg following STENDRA. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following STENDRA. One subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
A total of five subjects in Cohort B (tamsulosin) experienced potentially clinically important absolute values or changes from baseline in standing SBP or DBP. Two subjects experienced standing SBP values less than 85 mmHg following STENDRA. One subject experienced a decrease from baseline in standing SBP greater than 30 mmHg following STENDRA. Two subjects experienced standing DBP values less than 45 mmHg following STENDRA. Four subjects experienced decreases from baseline in standing DBP greater than 20 mmHg following STENDRA; one subject experienced such decreases following placebo. There were no severe adverse events related to hypotension reported during the trial. There were no cases of syncope.
Table 6 presents the placebo-subtracted mean maximum decreases from baseline (95% CI) in systolic blood pressure results for the 24 subjects who received STENDRA 200 mg and matching placebo.
Table 6: Placebo-Subtracted Mean (95% CI) Maximum Decreases from Baseline in Standing and Supine Systolic Blood Pressure (mmHg) with 200 mg STENDRA Doxazosin
Supine
Standing-6.0 (-9.1, -2.9)
-2.5 (-6.5, 1.5)Tamsulosin
Supine
Standing-3.1 (-6.4, 0.1)
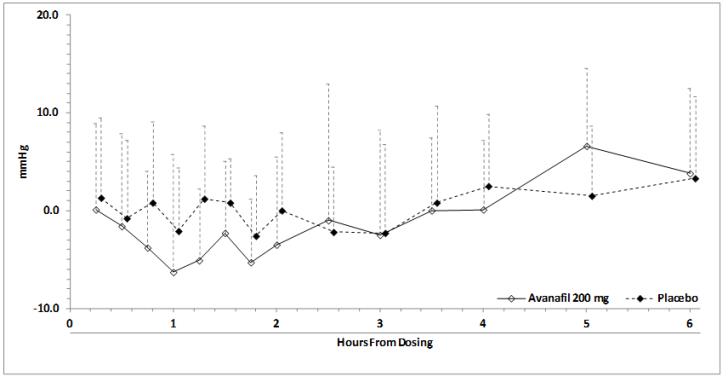
-3.6 (-8.1, 0.9)Blood pressure effects (standing SBP) in normotensive men on stable dose doxazosin (8 mg) following administration of STENDRA 200 mg or placebo, are shown in Figure 2. Blood pressure effects (standing SBP) in normotensive men on stable dose tamsulosin (0.4 mg) following administration of STENDRA 200 mg or placebo are shown in Figure 3.
Figure 2: Mean (SD) Change From Baseline in Standing Systolic Blood Pressure Over Time Following Administration of a Single Dose 200 mg Dose of STENDRA with Doxazosin
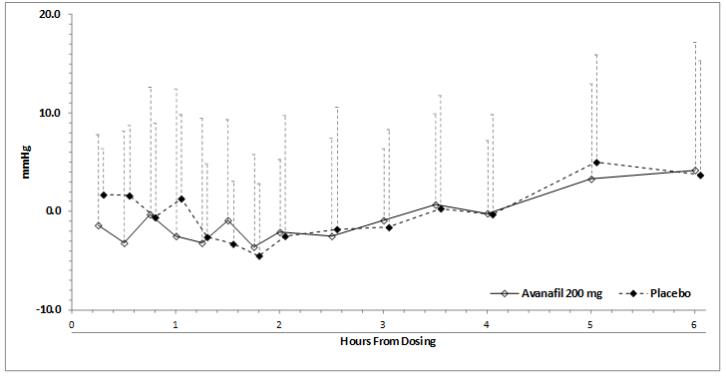
Figure 3: Mean (SD) Change From Baseline in Standing Systolic Blood Pressure Over Time Following Administration of a Single Dose 200 mg Dose of STENDRA with Tamsulosin
Effects of STENDRA on Blood Pressure When Administered with Enalapril
A trial was conducted to assess the interaction of enalapril (20 mg daily) and STENDRA 200 mg. Single doses of 200 mg STENDRA co-administered with enalapril caused a mean maximum decrease in supine systolic/diastolic blood pressure of 1.8/3.5 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm.
Effects of STENDRA on Blood Pressure When Administered with Amlodipine
A trial was conducted to assess the interaction of amlodipine (5 mg daily) and STENDRA 200 mg. Single doses of 200 mg STENDRA co-administered with amlodipine caused a mean maximum decrease in supine systolic blood pressure of 1.2 mmHg (compared to placebo), accompanied by a mean maximum increase in pulse rate of 1.0 bpm; the mean maximum decrease in diastolic blood pressure was less than that observed in the placebo group. There was no effect of STENDRA on amlodipine plasma concentrations. Concomitant amlodipine was associated with 22% and 70% increases in avanafil Cmax and AUC, respectively.
Effects of STENDRA on Blood Pressure When Administered with Alcohol
Alcohol and PDE5 inhibitors, including STENDRA, are mild systemic vasodilators. The interaction of STENDRA with alcohol was evaluated in a clinical pharmacology trial. Alcohol was administered at a dose of 0.5 g/kg, which is equivalent to approximately 3 ounces of 80-proof vodka in a 70-kg male, and STENDRA was administered at a dose of 200 mg. All patients consumed the entire alcohol dose within 15 minutes of starting. Blood alcohol levels of 0.057% were confirmed. There were no reports of orthostatic hypotension or dizziness. Additional maximum supine systolic/diastolic blood pressure decreases of 3.5/4.5 mmHg and additional maximum pulse rate increase of 9.3 bpm were observed when avanafil was taken with alcohol compared to alcohol alone. Avanafil did not affect alcohol plasma concentrations.
Effects of STENDRA on Spermatogenesis
The effect of STENDRA on spermatogenesis was assessed in 181 healthy male volunteers who received STENDRA 100 mg or placebo daily for 26 weeks. The results of this randomized, double-blind, placebo-controlled study in 137 subjects who completed the study through Week 26 and provided 2 semen samples at baseline and at Week 26 showed no adverse effects of STENDRA on sperm concentration, total sperm count, sperm motility, sperm normal morphology, and semen volume.
Effects of STENDRA on Vision
Single oral doses of Type 5 phosphodiesterase inhibitors have demonstrated transient dose-related impairment of color discrimination (blue/green), using the Farnsworth-Munsell 100-hue test, with peak effects near the time of peak plasma levels. This finding is consistent with the inhibition of PDE6, which is involved in phototransduction in the retina.
12.3 Pharmacokinetics
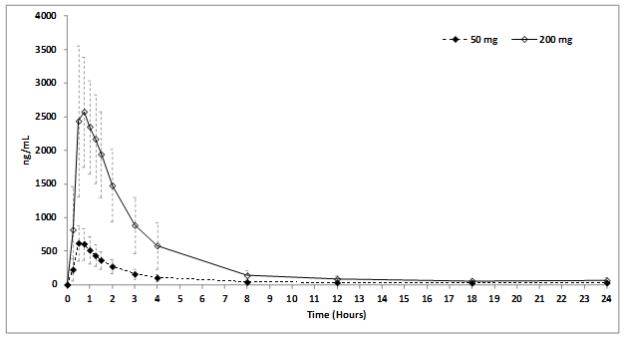
Mean STENDRA plasma concentrations measured after the administration of a single oral dose of 50 or 200 mg to healthy male volunteers are depicted in Figure 4. The pharmacokinetics of STENDRA are dose proportional from 12.5 to 600 mg.
Figure 4: Plasma Avanafil Concentrations (mean ± SD) Following a Single 50 mg or 200 mg STENDRA Dose
Absorption and Distribution
STENDRA is rapidly absorbed after oral administration, with a median Tmax of 30 to 45 minutes in the fasted state. When STENDRA (200 mg) is taken with a high fat meal, the rate of absorption is reduced, with a mean delay in Tmax of 1.12 to 1.25 hours and a mean reduction in Cmax of 39% (200 mg). There was an approximate 3.8% decrease in AUC. The small changes in avanafil Cmax and AUC are considered of minimal clinical significance; therefore, STENDRA may be administered with or without food. The mean accumulation ratio is approximately 1.2. Avanafil is approximately 99% bound to plasma proteins. Protein binding is independent of total drug concentrations, age, renal and hepatic function.
Based upon measurements of avanafil in semen of healthy volunteers 45-90 minutes after dosing, less than 0.0002% of the administered dose appeared in the semen of patients.
Metabolism and Excretion
Avanafil is cleared predominantly by hepatic metabolism, mainly by the CYP3A4 enzyme and to a minor extent by CYP2C isoform. The plasma concentrations of the major circulating metabolites, M4 and M16, are approximately 23% and 29% that of the parent compound, respectively. The M4 metabolite has an in vitro inhibitory potency for PDE5 18% of that of avanafil and M4 accounts for approximately 4% of the pharmacologic activity of avanafil. The M16 metabolite was inactive against PDE5.
Avanafil was extensively metabolized in humans. After oral administration, avanafil is excreted as metabolites predominantly in the feces (approximately 62% of administered oral dose) and to a lesser extent in the urine (approximately 21% of the administered oral dose). STENDRA has a terminal elimination half-life of approximately 5 hours.
Geriatric
The pharmacokinetics of a single 200 mg STENDRA administered to fourteen healthy elderly male volunteers (65-80 years) and eighteen healthy younger male volunteers (18-43 years of age) were compared. AUC0-inf increased by 6.8% and Cmax decreased by 2.1% in the elderly group, compared to the younger group. However, greater sensitivity to medications in some older individuals should be considered [see Use in Specific Populations (8.5)].
Renal Impairment
The pharmacokinetics of a single 200 mg STENDRA administered to nine patients with mild (creatinine clearance greater than or equal to 60 and less than 90 mL/min) and to ten patients with moderate (creatinine clearance greater than or equal to 30 to less than 60 mL/min) renal impairment were evaluated. AUC0-inf decreased by 2.9% and Cmax increased by 2.8% in patients with mild renal impairment, compared to healthy volunteers with normal renal function. AUC0-inf increased by 9.1% and Cmax decreased by 2.8% in patients with moderate renal impairment, compared to healthy volunteers with normal renal function. There is no data available for subjects with severe renal insufficiency or end-stage renal disease on hemodialysis [see Use in Specific Populations (8.6)].
Hepatic Impairment
The pharmacokinetics of a single 200 mg STENDRA administered to eight patients with mild hepatic impairment (Child-Pugh A) and eight patients with moderate hepatic impairment (Child-Pugh B) were evaluated. AUC0-inf increased by 3.8% and Cmax decreased by 2.7% in patients with mild hepatic impairment, compared to healthy volunteers with normal hepatic function. AUC0-inf increased by 11.2% and Cmax decreased by 51% in patients with moderate hepatic impairment, compared to healthy volunteers with normal hepatic function. There is no data available for subjects with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7)].
Drug Interaction Studies
Clinical Studies
Effect of CYP3A4 Inhibitors on Avanafil: Strong and moderate CYP3A4 inhibitors increase plasma concentrations of STENDRA. The effect of strong CYP3A4 inhibitors, ketoconazole and ritonavir, and moderate CYP3A4 inhibitor, erythromycin, on avanafil pharmacokinetics was studied in an open-label, randomized, one-sequence crossover, three-way parallel study.
Strong CYP3A4 Inhibitors
Fifteen healthy male volunteers received 400 mg ketoconazole (2 tablets containing 200 mg ketoconazole) once daily for 5 days (Days 2-6) and a single 50 mg avanafil on Days 1 and 6. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 6 were compared. Co-administration with the strong CYP3A4 inhibitor ketoconazole resulted in an approximate 13-fold increase in AUC0-inf and 3.1-fold increase in Cmax. Fourteen healthy male volunteers received 300 mg ritonavir (3 tablets containing 100 mg ritonavir) twice daily for 1 day (Day 2), 400 mg twice daily for 1 day (Day 3), 600 mg twice daily for 5 days (Days 4-8), and a single 50 mg avanafil on Days 1 and 8. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 8 were compared. Co-administration with the strong CYP3A4 inhibitor ritonavir resulted in an approximate 13-fold increase in AUC0-inf and 2.4-fold increase in Cmax of avanafil.
Moderate CYP3A4 Inhibitors
Fifteen healthy male volunteers received 500 mg erythromycin (2 tablets containing 250 mg erythromycin) every 12 hrs for 5 days (Days 2-6) and a single 200 mg avanafil (2 tablets containing 100 mg avanafil) on Days 1 and 6. Twenty-four hour pharmacokinetics of avanafil on Days 1 and 6 were compared. Co-administration with the moderate CYP3A4 inhibitor erythromycin resulted in an approximate 3.6-fold increase in AUC0-inf and 2.0-fold increase in Cmax of avanafil.
Effect of Avanafil on Other Drugs:
Warfarin
The effect of avanafil on warfarin pharmacokinetics and pharmacodynamics was evaluated in a double-blind, randomized, placebo-controlled, two-way crossover study. Twenty-four healthy male volunteers were randomized to receive either 200 mg avanafil or matching placebo for 9 days. On Day 3 of each period, volunteers received a single 25 mg warfarin. Pharmacokinetics of R- and S-warfarin, PT, and INR prior to warfarin dosing and up to 168 hrs after warfarin administration were compared. Platelet aggregation prior to warfarin dosing and up to 24 hrs after warfarin administration were compared. PT, INR, and platelet aggregation did not change with avanafil administration: 23.1 sec, 2.2, and 75.5%, respectively. Co-administration with avanafil resulted in an approximate 1.6% increase in AUC0-inf and 5.2% decrease in Cmax of S-warfarin.
Omeprazole, Rosiglitazone, and Desipramine
The effect of avanafil on the pharmacokinetics of omeprazole (a CYP2C19 substrate), rosiglitazone (a CYP2C8 substrate), and desipramine (a CYP2D6 substrate) was evaluated in an open-label, three cohort, crossover study. Nineteen healthy male volunteers received a single 40 mg omeprazole delayed-release capsule once daily for 8 days (Days 1-8), and a single 200 mg avanafil on Day 8. Twelve hour pharmacokinetics of omeprazole on Days 7 and 8 were compared. Co-administration with avanafil resulted in an approximate 5.9% increase in AUC0-inf and 8.6% increase in Cmax of omeprazole. Twenty healthy male volunteers received a single 8 mg rosiglitazone tablet then a single 200 mg avanafil. Twenty-four hour pharmacokinetics of rosiglitazone with and without avanafil were compared. Co-administration with avanafil resulted in an approximate 2.0% increase in AUC0-inf and 14% decrease in Cmax of rosiglitazone. Twenty healthy male volunteers received a single 50 mg desipramine tablet then a single 200 mg avanafil tablet 2 hours after desipramine. Ninety-six hour pharmacokinetics of desipramine with and without avanafil were compared. Co-administration with avanafil resulted in an approximate 5.7% increase in AUC0-inf and 5.2% increase in Cmax of desipramine.
In vitro studies
Avanafil had no effect on CYP1A1/2, 2A6, 2B6 and 2E1 (IC50 greater than 100 micromolar) and weak inhibitory effects toward other isoforms (CYP2C8, 2C9, 2C19, 2D6, 3A4). Major circulating metabolites of avanafil (M4 and M16) had no effect on CYPs 1A, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4. Avanafil and its metabolites (M4 and M16) are unlikely to cause clinically significant inhibition of CYPs 1A, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 or 3A4.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis and Impairment of Fertility
Carcinogenesis
Avanafil was not carcinogenic to CD-1 mice when administered daily at doses of 100, 200, or 600 mg/kg/day orally by gavage for at least 98 weeks (approximately 11 times the MRHD on an AUC basis) or to Sprague Dawley rats when administered daily at doses of 100, 300, or 1000 mg/kg/day orally by gavage for at least 100 weeks (approximately 8 times for males and 34 times for females above the MRHD on an AUC basis).
Mutagenesis
Avanafil was not genotoxic in a series of tests. Avanafil was not mutagenic in Ames assays. Avanafil was not clastogenic in chromosome aberration assays using Chinese hamster ovary and lung cells, or in vivo in the mouse micronucleus assay. Avanafil did not affect DNA repair when tested in the rat unscheduled DNA synthesis assay.
Impairment of Fertility
In a rat fertility and early embryonic development study administered 100, 300, or 1000 mg/kg/day for 28 days prior to pairing and continued until euthanasia for males, and 14 days prior to pairing to gestation day 7 for females, a decrease in fertility, no or reduced sperm motility, altered estrous cycles, and an increased percentage of abnormal sperm (broken sperm with detached heads) occurred at exposures in males approximately 11 times the human exposure at a dose of 200 mg. The altered sperm effects were reversible at the end of a 9-week drug-free period. Systemic exposure at the NOAEL (300 mg/kg/day) was comparable to the human AUC at the MRHD of 200 mg.
13.2 Animal Toxicology and/or Pharmacology
Repeated oral administration of avanafil in multiple species resulted in signs of centrally-mediated toxicity including ataxia, tremor, convulsion, hypoactivity, recumbency, and/or prostration at doses resulting in exposures approximately 5-8 times the MRHD based on Cmax and 8-30 times the MRHD based on AUC.
-
14
CLINICAL STUDIES
STENDRA was evaluated in four randomized, double-blind, placebo-controlled, parallel trials of 2 to 3 months in duration. STENDRA was taken as needed at doses of 50 mg, 100 mg, and 200 mg (Study 1) and 100 mg and 200 mg (Study 2, Study 3 and Study 4). Patients were instructed to take 1 dose of study drug approximately 30 minutes (Study 1 and Study 2) or approximately 15 minutes (Study 4) prior to initiation of sexual activity. Food and alcohol intake was not restricted.
In addition, a subset of patients from 2 of these trials were enrolled into an open-label extension trial. In the open-label extension trial, all eligible patients were initially assigned to avanafil 100 mg. At any point during the trial, patients could request to have their dose of avanafil increased to 200 mg or decreased to 50 mg based on their individual response to treatment.
The 3 primary outcome measures in Studies 1, 2 and 3 were the erectile function domain of the International Index of Erectile Function (IIEF) and Questions 2 and 3 from Sexual Encounter Profile (SEP). The IIEF is a 4-week recall questionnaire that was administered at baseline and at 4-week intervals during treatment. The IIEF erectile function domain has a 30-point total score, where the higher scores reflect better erectile function. The SEP included diary-based measures of erectile function. Patients recorded information regarding each sexual attempt made throughout the trial. Question 2 of the SEP asks "Were you able to insert your penis into your partners vagina?" Question 3 of the SEP asks "Did your erection last long enough for you to have successful intercourse?"
In Study 4, the primary efficacy variable was the per-subject proportion of sexual attempts that had an erectogenic effect within approximately 15 minutes following dosing, where an erectogenic effect was defined as an erection sufficient for vaginal penetration and that enabled satisfactory completion of sexual intercourse.
Results are shown from the three, Phase 3, randomized, double-blind, placebo-controlled, parallel studies, one in the general ED population (Study 1), another in the diabetic population with ED (Study 2), and another in the radical prostatectomy population (Study 3).
Results in the General ED Population (Study 1):
STENDRA was evaluated in 646 men with ED of various etiologies (organic, psychogenic, mixed), in a randomized, double-blinded, parallel, placebo controlled fixed dose trial of 3 months duration. The mean age was 55.7 years (range 23 to 88 years). The population was 85.6% White, 13.2% Black, 0.9% Asian, and 0.3% of other races. The mean duration of ED was approximately 6.5 years. STENDRA at doses of 50 mg, 100 mg, and 200 mg demonstrated statistically significant improvement in all 3 primary efficacy variables relative to placebo (see Table 7).
Table 7: Mean Change From Baseline for Primary Efficacy Variables in General ED Population (Study 1) Placebo
(N=155)STENDRA 50 mg
(N=154)STENDRA 100 mg
(N=157)STENDRA 200 mg
(N=156)IIEF EF Domain Score Endpoint 15.3 18.1 20.9 22.2 Change from baseline† 2.9 5.4 8.3 9.5 p-value* 0.0014 <0.0001 <0.0001 Vaginal Penetration (SEP2) Endpoint 53.8% 64.3% 73.9% 77.3% Change from baseline 7.1% 18.2% 27.2% 29.8% p-value* - 0.0009 <0.0001 <0.0001 Successful Intercourse (SEP3) Endpoint 27.0% 41.3% 57.1% 57.0% Change from baseline 14.1% 27.8% 43.4% 44.2% p-value* - 0.0002 <0.0001 <0.0001 † least-squares estimate from ANCOVA model * comparison to placebo for change from baseline Results in the ED Population with Diabetes Mellitus (Study 2)
STENDRA was evaluated in ED patients (n=390) with type 1 or type 2 diabetes mellitus in a randomized, double-blind, parallel, placebo-controlled fixed dose trial of 3 months in duration. The mean age was 58 years (range 30 to 78 years). The population was 80.5% White, 17.2% Black, 1.5% Asian, and 0.8% of other races. The mean duration of ED was approximately 6 years. In this trial, STENDRA at doses of 100 mg and 200 mg demonstrated statistically significant improvement in all 3 primary efficacy variables as measured by the erectile function domain of the IIEF questionnaire; SEP2 and SEP3 (see Table 8).
Table 8: Mean Change From Baseline for Primary Efficacy Variables in ED Population with Diabetes Mellitus (Study 2) Placebo
(N=127)STENDRA 100 mg
(N=126)STENDRA 200 mg
(N=126)IIEF EF Domain Score Endpoint 13.2 15.8 17.3 Change from baseline† 1.8 4.5 5.4 p-value* - 0.0017 <0.0001 Vaginal Penetration (SEP2) Endpoint 42.0% 54.0% 63.5% Change from baseline 7.5% 21.5% 25.9% p-value* - 0.0004 <0.0001 Successful Intercourse (SEP3) Endpoint 20.5% 34.4% 40.0% Change from baseline 13.6% 28.7% 34.0% p-value* - <0.0001 <0.0001 † least-squares estimate from ANCOVA model * comparison to placebo for change from baseline Results in the ED Population following Radical Prostatectomy (Study 3)
The efficacy of STENDRA was evaluated in Study 3 (NCT00895011), a randomized, double-blind, parallel, placebo-controlled fixed dose trial of 3 months in duration. For study inclusion, patients had to have ED following bilateral, nerve-sparing radical prostatectomy. Patients received STENDRA 100 mg or 200 mg once daily. A total of 286 patients were included in the efficacy population: mean age 58.4 years (range 40 to 70 years), 81.5% White, 18.1% Black and 0.3% of other races. Treatment with STENDRA at doses of 100 mg and 200 mg demonstrated statistically significant improvement in all 3 primary efficacy variables relative to placebo (see Table 9).
Table 9: Mean Change From Baseline for Primary Efficacy Variables in ED Population Following Radical Prostatectomy (Study 3) † least-squares estimates were obtained using an Analysis of Covariance (ANCOVA) model with treatment and baseline ED severity category as factors and baseline value as a covariate. * comparison to placebo for change from baseline
Placebo
(N=96)STENDRA 100 mg
(N=94)STENDRA 200 mg
(N=96)IIEF EF Domain Score Endpoint 9.3 12.6 14.7 Change from baseline† 1.2 4.7 6.2 p-value* - 0.0001 <0.0001 Vaginal Penetration (SEP2) Endpoint 19.7% 32.5% 40.8% Change from baseline† 7.5% 22.3% 27.7% p-value* - 0.0003 <0.0001 Successful Intercourse (SEP3) Endpoint 8.9% 23.4% 26.4% Change from baseline† 13.9% 28.0% 29.4% p-value* - 0.0004 <0.0001 Time to Onset of Effect (Study 4)
STENDRA was evaluated in 440 subjects with ED including diabetics (16.4%) and subjects with severe ED (41.4%) in a randomized double-blind, parallel, placebo-controlled study of 2 months duration. The mean age was 58.2 years (range 24 to 86 years). The population was 75.7% White, 21.4% Black, 1.6% Asian, and 1.4% of other races. Subjects were encouraged to attempt intercourse approximately 15 minutes after dosing and used a stopwatch for measurement of time to onset of effect, defined as the time to the first occurrence of an erection sufficient for sexual intercourse.
STENDRA 100 mg and 200 mg demonstrated statistically significant improvements relative to placebo in the primary efficacy variable, percentage of all attempts resulting in an erection sufficient for penetration at approximately 15 minutes after dosing followed by successful intercourse (SEP3) (see Table 10).
Table 10: Percentage of All Attempts Resulting in an Erection Sufficient for Penetration at Approximately 15 minutes After Dosing Followed by Successful Intercourse (SEP3) During the 8-Week Treatment Period in the Time to Onset of Effect (Study 4) Placebo
(N=136)STENDRA 100 mg
(N=139)STENDRA 200 mg
(N=139)Percentage of Successful Intercourse (SEP3) Mean 14.9 25.9 29.1 Median 0.0 11.1 13.3 p-value* - 0.001 <0.001 *comparison to placebo using rank-ANCOVA model.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
STENDRA (avanafil) is supplied as oval, pale yellow tablets containing 50 mg, 100 mg, or 200 mg avanafil debossed with dosage strengths.
50 mg 100 mg 200 mg Bottle of 30 NDC 72384-751-30 NDC 72384-752-30 NDC 72384-753-30 Recommended Storage: Store at 20-25°C (68-77°F); excursions permitted, 15-30°C (59-86°F) [see USP Controlled Room Temperature].
Protect from light.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
17.1 Nitrates
Physicians should discuss with patients the contraindication of STENDRA with regular and/or intermittent use of organic nitrates. Patients should be counseled that concomitant use of STENDRA with nitrates could cause blood pressure to suddenly drop to an unsafe level, resulting in dizziness, syncope, or even heart attack or stroke.
Physicians should discuss with patients the appropriate action in the event that they experience anginal chest pain requiring nitroglycerin following intake of STENDRA. In such a patient, who has taken STENDRA, where nitrate administration is deemed medically necessary in a life-threatening situation, at least 12 hours should elapse after the last dose of STENDRA before nitrate administration is considered. In such circumstances, nitrates should still only be administered under close medical supervision with appropriate hemodynamic monitoring. Patients who experience anginal chest pain after taking STENDRA should seek immediate medical attention [see Contraindications (4.1) and Warnings and Precautions (5.1)].
17.2 Cardiovascular Considerations
Physicians should discuss with patients the potential cardiac risk of sexual activity in patients with preexisting cardiovascular risk factors. Patients who experience symptoms upon initiation of sexual activity should be advised to refrain from further sexual activity and should seek immediate medical attention [see Warnings and Precautions (5.1)].
17.3 Concomitant Use with Drugs Which Lower Blood Pressure
Physicians should advise patients of the potential for STENDRA to augment the blood pressure-lowering effect of alpha-blockers and other antihypertensive medications [see Warnings and Precautions (5.6), Drug Interactions (7.1), and Clinical Pharmacology (12.2)].
17.4 Potential for Drug Interactions
Patients should be advised to contact the prescribing physician if new medications that may interact with STENDRA are prescribed by another healthcare provider.
17.5 Priapism
There have been rare reports of prolonged erections greater than 4 hours and priapism (painful erections greater than 6 hours in duration) for this class of compounds. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Physicians should advise patients who have an erection lasting greater than 4 hours, whether painful or not, to seek emergency medical attention.
17.6 Vision
Physicians should advise patients to stop use of all PDE5 inhibitors, including STENDRA, and seek medical attention in the event of a sudden loss of vision in one or both eyes. Such an event may be a sign of non-arteritic anterior ischemic optic neuropathy (NAION), a cause of decreased vision including permanent loss of vision that has been reported rarely in temporal association with the use of PDE5 inhibitors. Physicians should discuss with patients the increased risk of NAION in individuals who have already experienced NAION in one eye. Physicians should also discuss with patients the increased risk of NAION among the general population in patients with a “crowded” optic disc, although evidence is insufficient to support screening of prospective users of PDE5 inhibitor, including STENDRA, for these uncommon conditions [see Warnings and Precautions (5.4) and Adverse Reactions (6.2)].
17.7 Sudden Hearing Loss
Physicians should advise patients to stop taking PDE5 inhibitors, including STENDRA, and seek prompt medical attention in the event of sudden decrease or loss of hearing. Use of PDE5 inhibitors has been associated with sudden decrease or loss of hearing, which may be accompanied by tinnitus and dizziness. It is not possible to determine whether these events are related directly to the use of PDE5 inhibitors or to other factors [see Adverse Reactions (6)].
17.8 Alcohol
Patients should be made aware that both alcohol and PDE5 inhibitors including STENDRA act as mild vasodilators. When mild vasodilators are taken in combination, blood pressure-lowering effects of each individual compound may be increased. Therefore, physicians should inform patients that substantial consumption of alcohol (e.g., greater than 3 units) in combination with STENDRA can increase the potential for orthostatic signs and symptoms, including increase in heart rate, decrease in standing blood pressure, dizziness, and headache [see Warnings and Precautions (5.7) and Clinical Pharmacology (12.2)].
17.9 Sexually Transmitted Disease
The use of STENDRA offers no protection against sexually transmitted diseases. Counseling of patients about the protective measures necessary to guard against sexually transmitted diseases, including Human Immunodeficiency Virus (HIV) should be considered.
17.10 Recommended Administration
Physicians should discuss with patients the appropriate use of STENDRA and its anticipated benefits. It should be explained that sexual stimulation is required for an erection to occur after taking STENDRA. Patients should be counseled regarding the dosing of STENDRA. Inform patients that the recommended starting dose of STENDRA is 100 mg, taken as early as approximately 15 minutes before initiation of sexual activity. Based on efficacy and tolerability, the dose may be increased to 200 mg taken as early as approximately 15 minutes before sexual activity, or decreased to 50 mg taken approximately 30 minutes before sexual activity. The lowest dose that provides benefit should be used. Patients should be advised to contact their healthcare provider for dose modification.
17.11 Guanylate Cyclase (GC) Stimulators
Physicians should discuss with patients the contraindication of STENDRA with use of guanylate cyclase stimulators such as riociguat or vericiguat [see Contraindications (4.3)].
Manufactured for: Metuchen Pharmaceuticals, LLC, Freehold, NJ 07728
By: Sanofi Winthrope Industrie, Ambares, France© Metuchen Pharmaceuticals, LLC. All rights reserved.
US Patent Number: 6,656,935 and 7,501,409
STENDRA is a registered U.S. trademark of Metuchen Pharmaceuticals, LLC.
375F101 -
PATIENT PACKAGE INSERT
Patient Information STENDRA® (sten-druh)
(avanafil)
tablets, for oral use
What is the most important information I should know about STENDRA?
STENDRA can cause your blood pressure to drop suddenly to an unsafe level if it is taken with certain other
medicines. Do not take STENDRA if you take any medicines called nitrates. Nitrates are used to treat chest pain
(angina). A sudden drop in blood pressure can cause you to feel dizzy, faint, or have a heart attack or stroke.
Do not take STENDRA if you take medicines called guanylate cyclase stimulators which include:
• Riociguat and vericiguat, medicines that treat pulmonary arterial hypertension and chronic-thromboembolicpulmonary hypertension.
Ask your healthcare provider or pharmacist if any of your medicines are nitrates or guanylate cyclase stimulators.
Tell all your healthcare providers that you take STENDRA. If you need emergency medical care for a heartproblem, it will be important for your healthcare provider to know when you last took STENDRA.
Stop sexual activity and get medical help right away if you get symptoms such as chest pain, dizziness, or nausea
during sex. Sexual activity can put an extra strain on your heart, especially if your heart is already weak from a heart
attack or heart disease.
What is STENDRA?
STENDRA is a prescription medicine used to treat erectile dysfunction (ED) in adult men.
STENDRA is not for use in women or children.
It is not known if STENDRA is safe and effective in women or children under 18 years of age.
Do not take STENDRA if you:
• take medicines called nitrates.
• use street drugs called “poppers” such as amyl nitrite and butyl nitrite.
• are allergic to avanafil or any of the ingredients in STENDRA. See the end of this leaflet for a complete list ofingredients in STENDRA.
• take any medicines called guanylate cyclase stimulators.
Before you take STENDRA, tell your healthcare provider about all of your medical conditions, including if you:
• have or have had heart problems such as a heart attack, irregular heartbeat, angina, or heart failure.
• have had heart surgery within the last 6 months.
• have had a stroke.
• have low blood pressure, or high blood pressure that is not controlled.
• have a deformed penis shape.
• have had an erection that lasted for more than 4 hours.
• have problems with your blood cells such as sickle cell anemia, multiple myeloma, or leukemia.
• have retinitis pigmentosa, a rare genetic (runs in families) eye disease.
• have ever had severe vision loss, including an eye problem called non-arteritic anterior ischemic optic neuropathy(NAION).
• have had a sudden decrease or loss of hearing.
• have bleeding problems.
• have or have had stomach ulcers.
• have liver problems.
• have kidney problems or are having kidney dialysis.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter
medicines, vitamins, and herbal supplements.
STENDRA may affect the way other medicines work, and other medicines may affect the way STENDRA works
causing side effects. Especially tell your healthcare provider if you take any of the following:
• medicines called nitrates (see What is the most important information I should know about STENDRA?)
• medicines called guanylate cyclase stimulators, such a riociguat or vericiguat (see What is the most importantinformation I should know about STENDRA?)
• medicines called HIV protease inhibitors.
• some types of oral antifungal medicines, such as ketoconazole, and itraconozale.
• some types of antibiotics, such as clarithromycin, telithromycin, or erythromycin.
• medicines called alpha blockers. These include terazosin, tamsulosin HCl, doxazosin, prazosin HCl, alfuzosin HCl,
dutasteride and tamsulosin HCl, or silodosin. Alpha-blockers are sometimes prescribed for prostate problems or
high blood pressure. In some patients, the use of STENDRA with alpha-blockers can lead to a drop in blood
pressure or to fainting.
• other medicines that treat high blood pressure.
• other medicines or treatments for ED.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a
new medicine.
How should I take STENDRA?
• Take STENDRA exactly as your healthcare provider tells you to take it.
• Your healthcare provider will tell you how much STENDRA to take and when to take it.
• Take STENDRA 100 mg or 200 mg as early as 15 minutes before sexual activity or
• Take STENDRA 50 mg as early as 30 minutes before sexual activity.• Do not take STENDRA more than 1 time a day.
• Some form of sexual stimulation is needed for an erection to happen after taking STENDRA.
• Your healthcare provider may change your dose if needed.
• You should take the lowest dose of STENDRA that works for you. You and your healthcare provider should decide
about the lowest dose of STENDRA that works for you.
• STENDRA may be taken with or without food.
• If you take too much STENDRA, call your healthcare provider or go to the nearest hospital emergency room rightaway.
What should I avoid while taking STENDRA?
• Do not drink too much alcohol when taking STENDRA (for example, 3 glasses of wine, or 3 shots of whiskey).
Drinking too much alcohol when taking STENDRA can increase your chances of increasing your heart rate,
lowering your blood pressure, dizziness or getting a headache.
What are the possible side effects of STENDRA?
STENDRA can cause serious side effects, including:
• See “What is the most important information I should know about STENDRA?”
• an erection that will not go away (priapism). If you have an erection that lasts more than 4 hours, get medical
help right away. If it is not treated right away, priapism can permanently damage your penis.
• sudden vision loss in 1 or both eyes. Sudden vision loss in 1 or both eyes can be a sign of a serious eye
problem called non-arteritic anterior ischemic optic neuropathy (NAION). It is uncertain whether PDE5 inhibitors
such as STENDRA, directly cause vision loss. Stop taking STENDRA and call your healthcare provider right away
if you have sudden vision loss in 1 or both eyes.
• sudden hearing decrease or hearing loss. Some people may also have ringing in their ears (tinnitus) or
dizziness.
The most common side effects of STENDRA are:
• headache
• flushing
• stuffy or runny nose
• sore throat
• back pain
These are not all of the possible side effects of STENDRA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store STENDRA?
• Store STENDRA at room temperature between 68°F to 77°F (20°C to 25°C).
• Keep STENDRA out of the light.
Keep STENDRA and all medicines out of the reach of children
General information about the safe and effective use of STENDRA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use
STENDRA for a condition for which it was not prescribed. Do not give STENDRA to other people, even if they have
the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for
information about STENDRA that is written for health professionals.
What are the ingredients in STENDRA?
Active ingredient: avanafil. Inactive ingredients: mannitol, fumaric acid, hydroxypropylcellulose, low substituted
hydroxypropylcellulose, calcium carbonate, magnesium stearate, and ferric oxide yellow.
Manufactured for: Metuchen Pharmaceuticals, LLC, Freehold, NJ 07728By: Sanofi Winthrope Industrie, Ambares, France;
© Metuchen Pharmaceuticals, LLC. All rights reserved.
US Patent Number: 6,656,935 and 7,501,409
STENDRA is a registered U.S. trademark of Metuchen Pharmaceuticals, LLC.
375P100
For more information, go to www.STENDRA.com or call 1-844-458-4887
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: October/2022
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
STENDRA
avanafil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72384-751 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 50 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) CALCIUM CARBONATE (UNII: H0G9379FGK) MAGNESIUM STEARATE (UNII: 70097M6I30) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (pale yellow) Score no score Shape OVAL Size 7mm Flavor Imprint Code 50mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72384-751-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/27/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202276 12/27/2013 STENDRA
avanafil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72384-752 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 100 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) CALCIUM CARBONATE (UNII: H0G9379FGK) MAGNESIUM STEARATE (UNII: 70097M6I30) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (pale yellow) Score no score Shape OVAL Size 9mm Flavor Imprint Code 100mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72384-752-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/27/2013 2 NDC:72384-752-99 6 in 1 CARTON 08/10/2020 2 NDC:72384-752-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202276 12/27/2013 STENDRA
avanafil tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72384-753 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVANAFIL (UNII: DR5S136IVO) (AVANAFIL - UNII:DR5S136IVO) AVANAFIL 200 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) FUMARIC ACID (UNII: 88XHZ13131) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) LOW-SUBSTITUTED HYDROXYPROPYL CELLULOSE (11% HYDROXYPROPYL; 130000 MW) (UNII: 7773C1ROEU) CALCIUM CARBONATE (UNII: H0G9379FGK) MAGNESIUM STEARATE (UNII: 70097M6I30) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (pale yellow) Score no score Shape OVAL Size 12mm Flavor Imprint Code 200mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72384-753-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/27/2013 2 NDC:72384-753-99 6 in 1 CARTON 08/10/2020 2 NDC:72384-753-01 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202276 12/27/2013 Labeler - Metuchen Pharmaceuticals, LLC (080405758)






