Label: ZORYVE- roflumilast aerosol, foam
- NDC Code(s): 80610-430-60
- Packager: Arcutis Biotherapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZORYVE foam, 0.3%, safely and effectively. See full prescribing information for ZORYVE foam, 0.3%. ZORYVE® (roflumilast) topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Seborrheic Dermatitis - ZORYVE® topical foam, 0.3%, is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older. 1.2 Plaque ...
-
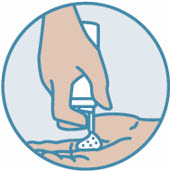
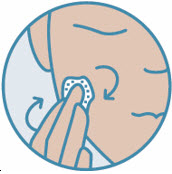
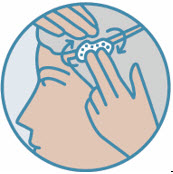
2 DOSAGE AND ADMINISTRATIONShake can prior to each use. Apply a thin layer of ZORYVE foam, 0.3%, once daily to affected areas of body and/or scalp when they are not wet. Rub in completely. Wash hands after ...
-
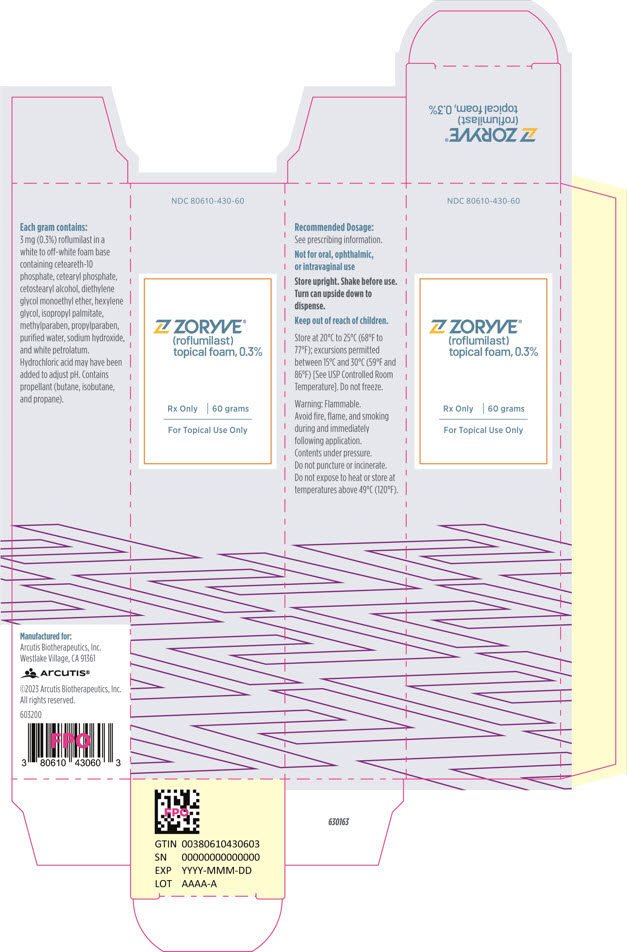
3 DOSAGE FORMS AND STRENGTHSTopical foam, 0.3%: 3 mg of roflumilast per gram of white to off-white foam in 60-gram pressurized cans.
-
4 CONTRAINDICATIONSZORYVE foam, 0.3%, is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C) [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - The propellants in ZORYVE foam, 0.3%, are flammable. Avoid fire, flame, and smoking during and immediately following application.
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on ZORYVE Foam, 0.3% Drugs that Inhibit Cytochrome P450 (CYP) Enzymes - No formal drug-drug interaction studies were conducted with ZORYVE foam, 0.3%; however, the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data available on the use of ZORYVE foam, 0.3%, in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or ...
-
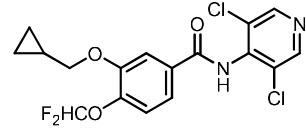
11 DESCRIPTIONZORYVE (roflumilast) topical foam, 0.3%, is a white to off-white foam for topical use. The active ingredient, roflumilast, is a phosphodiesterase 4 (PDE4) inhibitor. The chemical name of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Roflumilast and its active metabolite (roflumilast N-oxide) are inhibitors of PDE4. Roflumilast and roflumilast N-oxide inhibition of PDE4 (a major cyclic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies were conducted in hamsters and mice with roflumilast to evaluate its carcinogenic potential. In 2-year oral gavage ...
-
14 CLINICAL STUDIES14.1 Seborrheic Dermatitis - Two randomized, double-blind, vehicle-controlled trials (STRATUM [NCT04973228] and Trial 203 [NCT04091646]) enrolled a total of 683 adult and pediatric subjects with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ZORYVE (roflumilast) topical foam, 0.3%, is a white to off-white foam. It is supplied in a 60-gram pressurized aluminum can (NDC 80610-430-60). Storage and Handling - Store ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Patient Information). Administration Instructions - Advise patients or caregivers that ZORYVE foam, 0.3%, is for ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Arcutis Biotherapeutics, Inc. Westlake Village, CA 91361 - For more information on ZORYVE foam, call 1-805-418-5006 or visit http://www.zoryve.com. © 2025 Arcutis Biotherapeutics, Inc ...
-
PATIENT PACKAGE INSERTPatient Information - ZORYVE® (zor-EEV) (roflumilast) topical foam, 0.3% This Patient Information has been approved by the U.S. Food and Drug Administration.Revised: 5/2025 - Important ...
-
INSTRUCTIONS FOR USEZORYVE® (zor-EEV)(roflumilast) topical foam, 0.3%This Instructions for Use contains information on how to apply ZORYVE foam. Read this Instructions for Use before you start using ZORYVE foam and each time you get a refill. There may be new ...
-
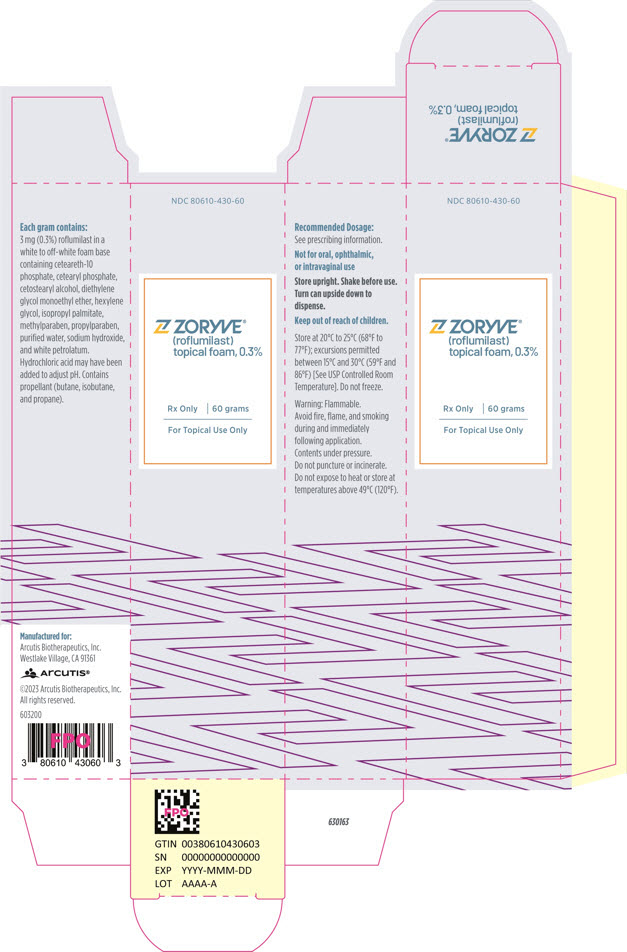
PRINCIPAL DISPLAY PANEL - 60 g Can CartonNDC 80610-430-60 - ZORYVE® (roflumilast) topical foam, 0.3% Rx Only - 60 grams - For Topical Use Only
-
INGREDIENTS AND APPEARANCEProduct Information