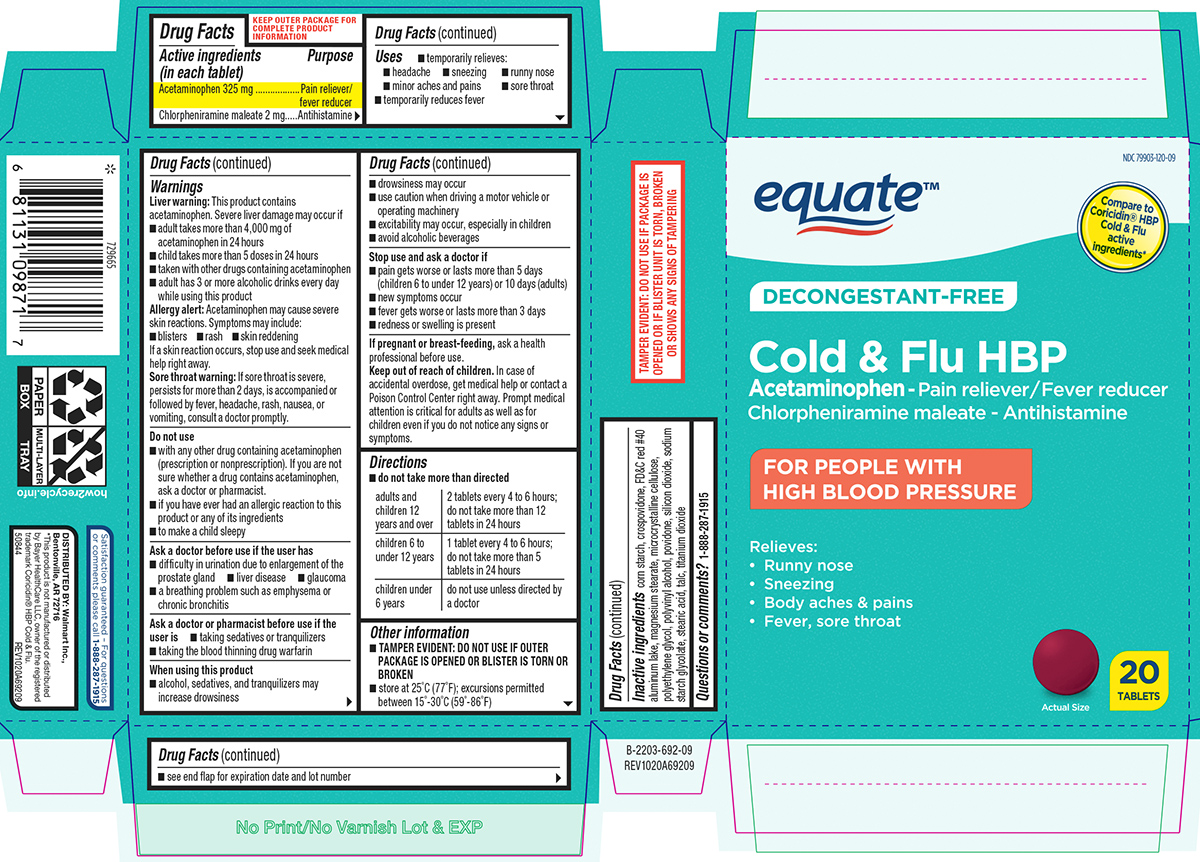
Label: COLD AND FLU HBP- acetaminophen, chlorpheniramine maleate tablet, film coated
- NDC Code(s): 79903-120-09
- Packager: WALMART INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- adult takes more than 4,000 mg of acetaminophen in 24 hours
- child takes more than 5 doses in 24 hours
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- blisters
- rash
- skin reddening
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
- to make a child sleepy
Ask a doctor before use if the user has
- difficulty in urination due to enlargement of the prostate gland
- liver disease
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if the user is
- taking sedatives or tranquilizers
- taking the blood thinning drug warfarin
When using this product
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- drowsiness may occur
- avoid alcoholic beverages
-
Directions
- do not take more than directed
adults and children 12 years and over 2 tablets every 4 to 6 hours; do not take more than 12 tablets in 24 hours children 6 to under 12 years 1 tablet every 4 to 6 hours; do not take more than 5 tablets in 24 hours children under 6 years do not use unless directed by a doctor - Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
equate™
NDC 79903-120-09
Compare to
Coricidin® HBP
Cold & Flu
active
ingredients*DECONGESTANT-FREE
Cold & Flu HBP
Acetaminophen - Pain reliever/Fever reducer
Chlorpheniramine maleate - AntihistamineFOR PEOPLE WITH
HIGH BLOOD PRESSURERelieves:
• Runny nose
• Sneezing
• Body aches & pains
• Fever, sore throatActual Size
20
TABLETSTAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS
TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERINGSatisfaction guaranteed – For questions
or comments please call 1-888-287-1915.DISTRIBUTED BY: Walmart Inc.,
Bentonville, AR 72716*This product is not manufactured or distributed by
Bayer HealthCare LLC, owner of the registered
trademark Coricidin® HBP Cold & Flu.
50844 ORG102069209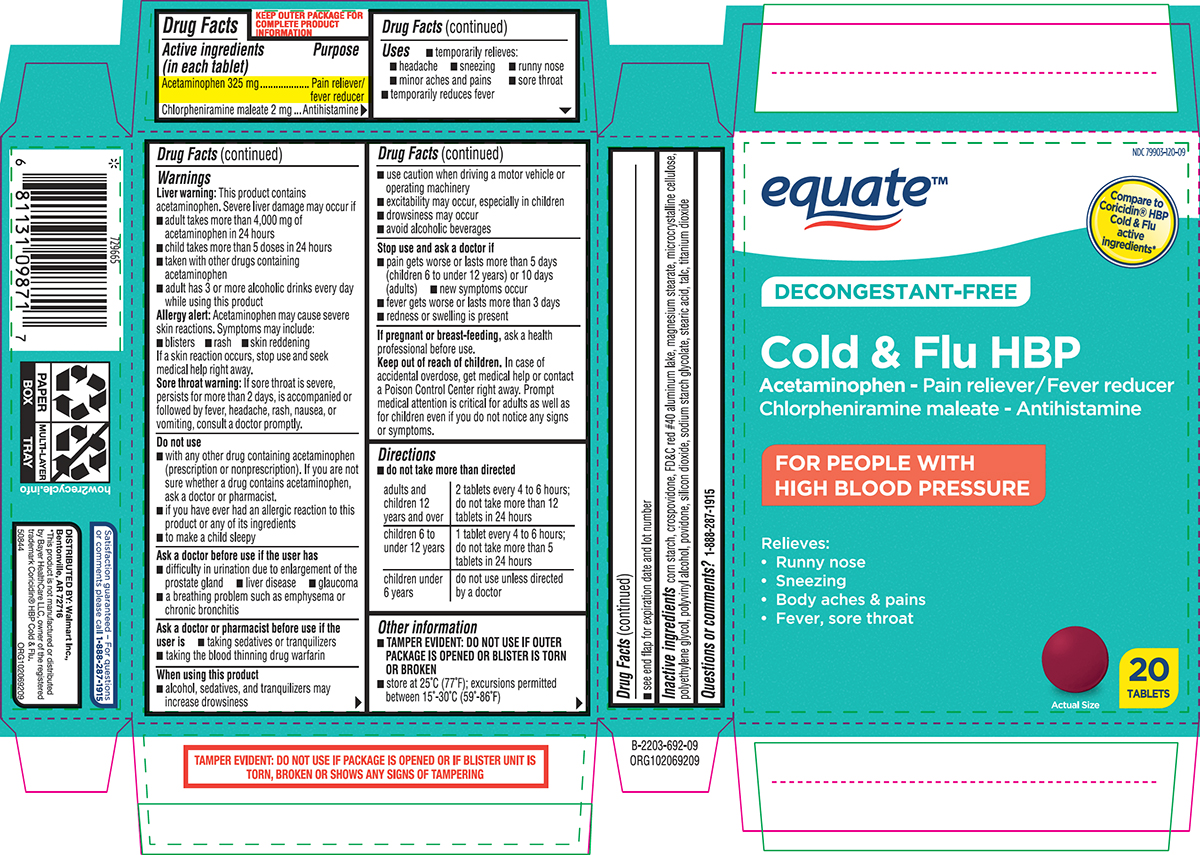
Equate 44-692
-
INGREDIENTS AND APPEARANCE
COLD AND FLU HBP
acetaminophen, chlorpheniramine maleate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-120 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) CROSPOVIDONE (UNII: 2S7830E561) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color red Score no score Shape ROUND Size 19mm Flavor Imprint Code 44;692 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-120-09 2 in 1 CARTON 04/14/2022 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/14/2022 Labeler - WALMART INC. (051957769) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(79903-120) , pack(79903-120) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 manufacture(79903-120) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(79903-120)