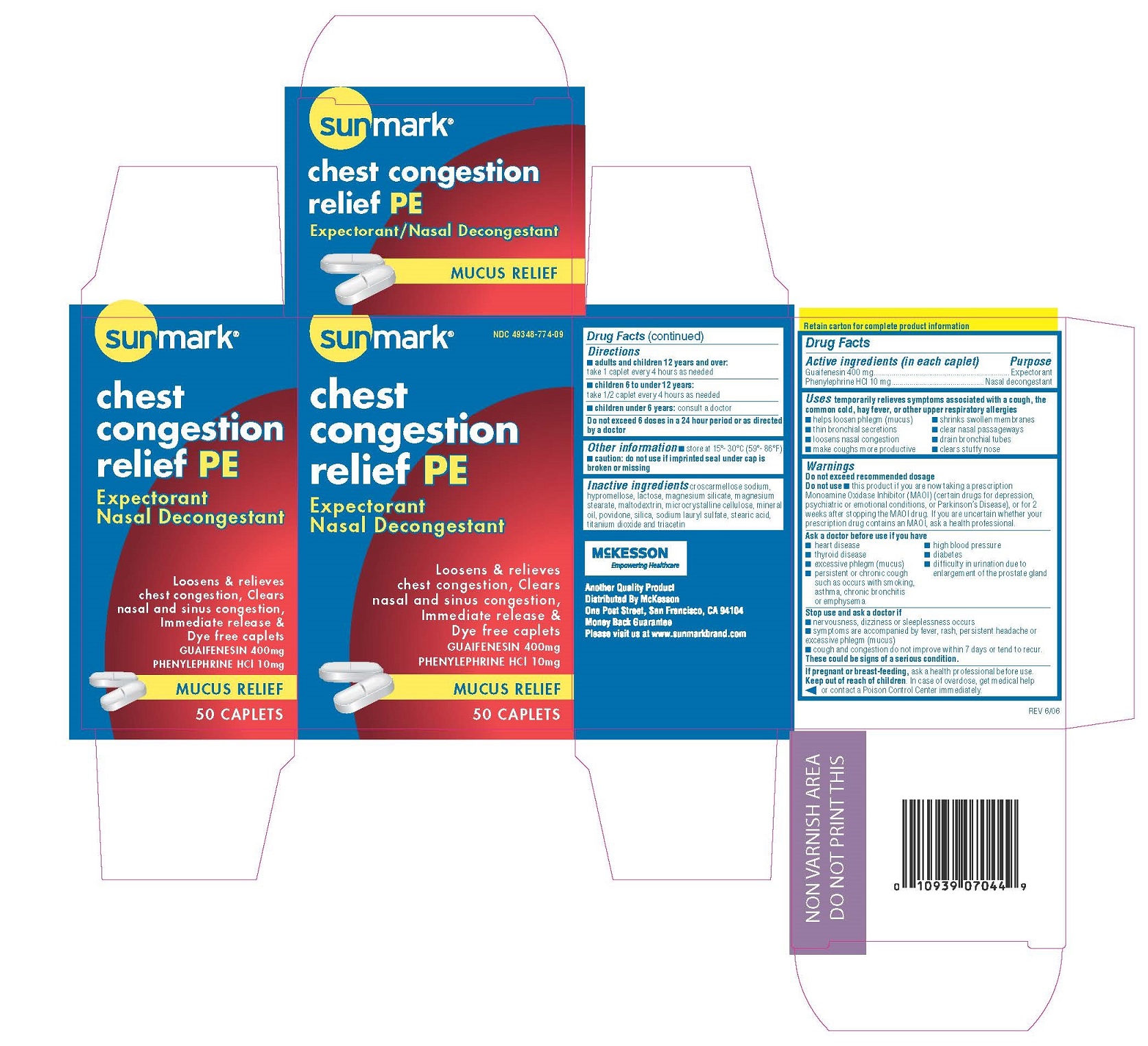
Label: SUNMARK CHEST CONGESTION RELIEF PE PE- guaifenesin/phenylephrine tablet
- NDC Code(s): 49348-774-09
- Packager: Strategic Sourcing Services LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive ingredient - (per tablet) Guaifenesin 400 mg - Phenylephrine HCl 10 mg
-
PurposeGuaifenesin.......................Expectorant - Phenylephrine HCl..............Nasal decongestant
-
UsesTemporarily relieves symptoms associated with a cough ,the common cold,hay fever or other upper respiratory allergies. ■ helps loosen phlegm (mucus) ■ clear nasal passageways - ■ loosens nasal ...
-
WarningsDo not exceed recommended dosage
-
Do not use■ this product if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks ...
-
Ask a doctor before use if you have■ heart disease - ■ high blood pressure - ■ thyroid disease - ■ diabetes - ■ excessive phlegm;mucus - ■ difficulty in urination due to an enlarged prostate gland - ■ persistent or chronic cough such as occurs ...
-
Stop use and ask a doctor if■ nervousness, dizziness or sleeplessness occurs - ■ symptoms are accompanied by fever, rash, persistent headache or excessive phlegm (mucus) ■ cough and congestion do not improve within 7 days - or ...
-
PREGNANCY OR BREAST FEEDINGIf pregnant or breast-feeding, ask a health - professional before use.
-
KEEP OUT OF REACH OF CHILDRENKeep out of the reach of children. In case of overdose, get medical help or contact a Poison - Control Center immediately.
-
Directions■ adults and children 12 years and over: take 1 caplet every 4 hours as needed - ■ children 6 to under 12 years: take 1/2 caplet every 4 hours as needed - ■ children under 6 years: consult a ...
-
Inactive ingredientslactose, magnesium silicate, croscarmellose sodium, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil, povidone, silica, sodium lauryl sulfate, stearic acid ...
-
PRINCIPAL DISPLAY PANEL
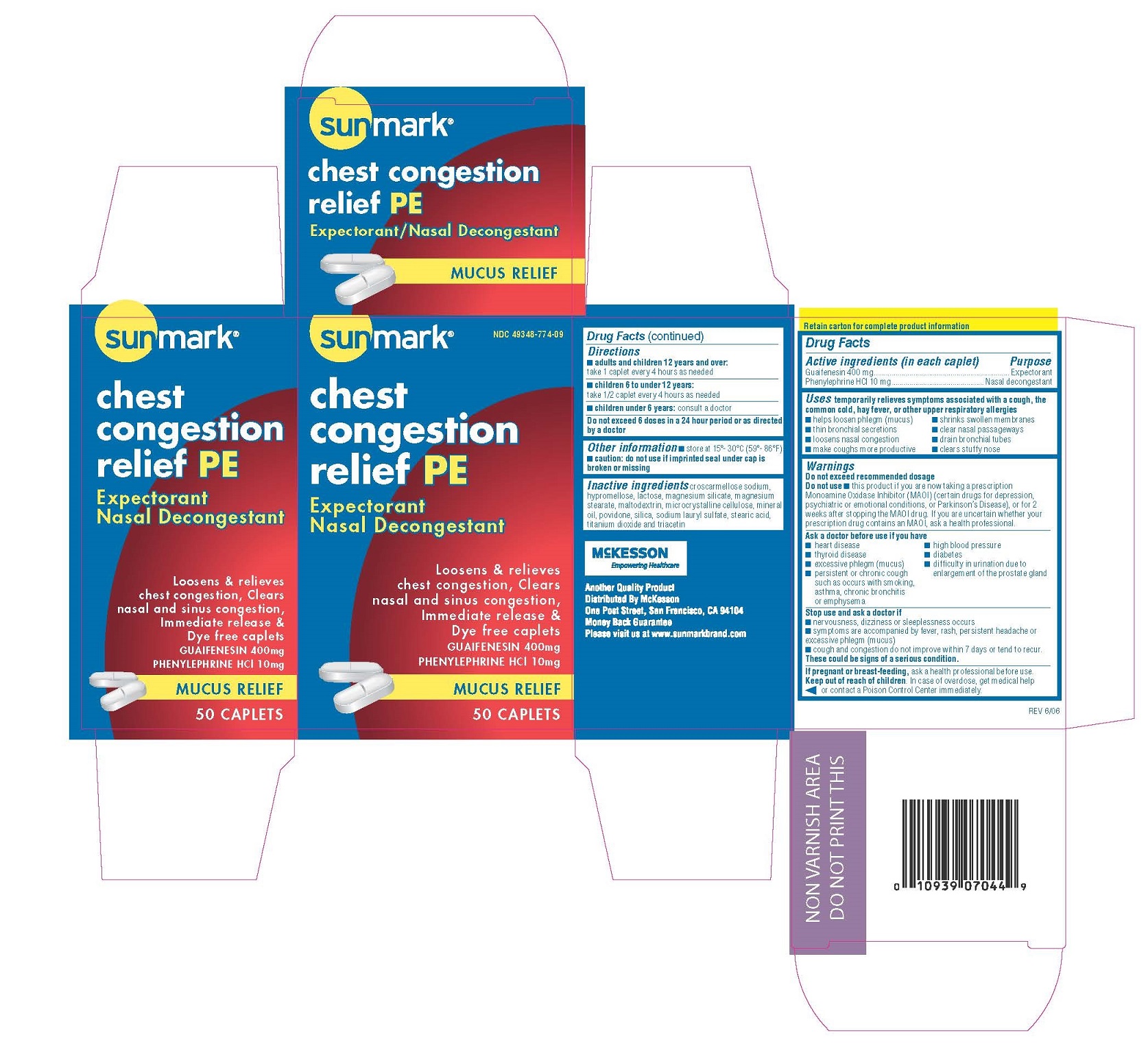
-
INGREDIENTS AND APPEARANCEProduct Information