Label: NIMODIPINE- nimodipine solution
- NDC Code(s): 31722-039-47
- Packager: Camber Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use NIMODIPINE ORAL SOLUTION safely and effectively. See full prescribing information for NIMODIPINE ORAL SOLUTION. NIMODIPINE oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USENimodipine oral solution is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration Instructions - Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. For all routes ...
-
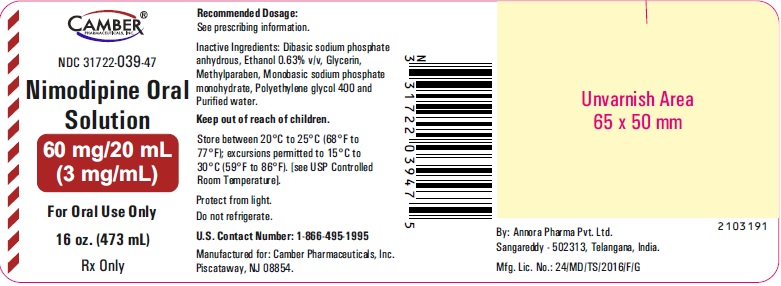
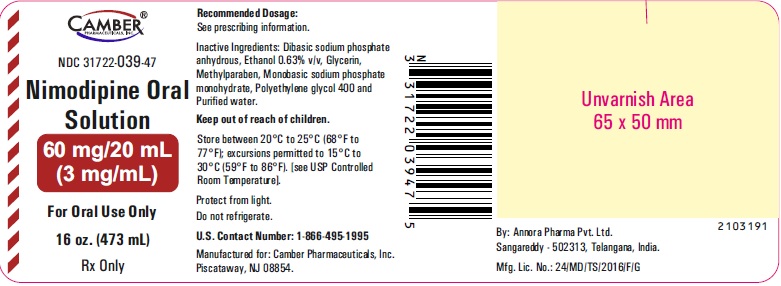
3 DOSAGE FORMS AND STRENGTHSOral Solution (3 mg per mL): • 60 mg/20 mL (3 mg/mL), pale yellow solution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - Blood pressure should be carefully monitored during treatment with nimodipine. In clinical studies of patients with subarachnoid hemorrhage, about 5% of nimodipine-treated ...
-
6 ADVERSE REACTIONSThe safety and efficacy of nimodipine oral solution in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH ...
-
7 DRUG INTERACTIONS7.1 Blood Pressure Lowering Drugs - Nimodipine may increase the blood pressure lowering effect of concomitantly administered anti-hypertensives such as diuretics, beta-blockers, ACE inhibitors ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate data on the developmental risk associated with the use of nimodipine in pregnant women. In animal studies, oral administration of ...
-
10 OVERDOSAGEThere have been no reports of overdosage from the oral administration of nimodipine. Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral ...
-
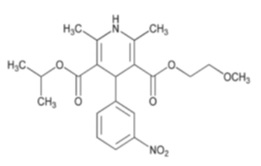
11 DESCRIPTIONNimodipine oral solution contains nimodipine, a dihydropyridine calcium channel blocker. Nimodipine is isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4- ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Nimodipine is a dihydropyridine calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a two-year study in rats, the incidences of adenocarcinoma of the uterus and Leydig cell adenoma of the testes ...
-
14 CLINICAL STUDIESThe safety and efficacy of nimodipine oral solution in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGNimodipine Oral Solution 3 mg/mL is a pale yellow solution and is supplied as follows: · NDC 31722-039-47: 16 oz. bottle (473 mL) 60 mg/20 mL (3 mg/mL). Store between 20°C to 25°C ...
-
17 PATIENT COUNSELING INFORMATIONInform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure - [see Warnings and Precautions - (5.1)]. Inform them that use of ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNimodipine Oral Solution 60 mg/20 mL Container Label
-
INGREDIENTS AND APPEARANCEProduct Information