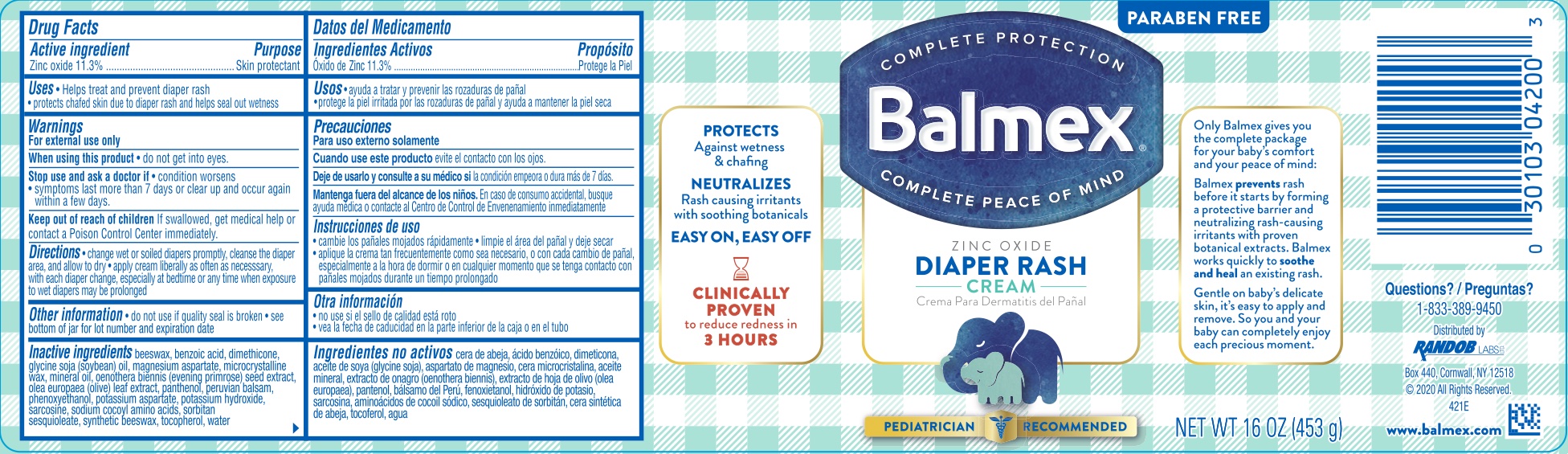
Label: BALMEX DIAPER RASH- zinc oxide 11.3% cream
-
NDC Code(s):
52412-285-02,
52412-285-03,
52412-285-04,
52412-285-12, view more52412-285-16
- Packager: Randob Labs, LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Beeswax, benzoic acid, dimethicone, glycine soja (soybean) oil, magnesium aspartate, microcrystalline wax, mineral oil, oenothera biennis (evening primrose) seed extract, olea europae (olive) leaf extract, panthenol, Peruvian balsam, Phenoxyethanol, potassium aspartate, potassium hydroxide, sarcosine, sodium cocoyl amino acids, sorbitan sesquioleate, synthetic beeswax, tocopherol, water.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BALMEX DIAPER RASH
zinc oxide 11.3% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52412-285 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 11.3 g in 100 g Inactive Ingredients Ingredient Name Strength BENZOIC ACID (UNII: 8SKN0B0MIM) DIMETHICONE 350 (UNII: 2Y53S6ATLU) SOYBEAN OIL (UNII: 241ATL177A) MAGNESIUM ASPARTATE (UNII: R17X820ROL) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) MINERAL OIL (UNII: T5L8T28FGP) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) OLEA EUROPAEA LEAF (UNII: MJ95C3OH47) PANTHENOL (UNII: WV9CM0O67Z) BALSAM PERU (UNII: 8P5F881OCY) PHENOXYETHANOL (UNII: HIE492ZZ3T) POTASSIUM ASPARTATE (UNII: OC4598NZEQ) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SARCOSINE (UNII: Z711V88R5F) COCO ACID CHLORIDES (UNII: R7N8Y0XMX7) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) YELLOW WAX (UNII: 2ZA36H0S2V) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52412-285-16 453 g in 1 JAR; Type 0: Not a Combination Product 04/19/2021 2 NDC:52412-285-12 340 g in 1 JAR; Type 0: Not a Combination Product 04/19/2021 3 NDC:52412-285-04 1 in 1 CARTON 04/19/2021 3 113 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:52412-285-02 1 in 1 CARTON 04/19/2021 4 56 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:52412-285-03 1 in 1 CARTON 04/04/2023 5 85 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 04/19/2021 Labeler - Randob Labs, LTD (061995007) Registrant - Derma Care Research Labs (116817470) Establishment Name Address ID/FEI Business Operations Derma Care Research Labs 116817470 manufacture(52412-285)