Label: RHOFADE- oxymetazoline hydrochloride cream
- NDC Code(s): 51862-765-30
- Packager: Mayne Pharma
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use RHOFADE® topical cream safely and effectively. See full prescribing information for RHOFADE® topical cream. RHOFADE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGERHOFADE® (oxymetazoline hydrochloride) cream, 1% is indicated for the topical treatment of persistent facial erythema associated with rosacea in adults.
-
2 DOSAGE AND ADMINISTRATIONFor topical use only. RHOFADE cream is not for oral, ophthalmic, or intravaginal use. Apply a pea-sized amount of RHOFADE cream, once daily in a thin layer to cover the entire face (forehead ...
-
3 DOSAGE FORMS AND STRENGTHSRHOFADE® (oxymetazoline hydrochloride) cream, 1% is a white to off-white cream. Each gram of cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Potential Impacts on Cardiovascular Disease - Alpha-adrenergic agonists may impact blood pressure. RHOFADE cream should be used with caution in patients with severe or unstable or ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to ...
-
7 DRUG INTERACTIONS7.1 Anti-hypertensives/Cardiac Glycosides - Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives and/or cardiac ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on RHOFADE cream use in pregnant women to inform a drug-associated risk for major birth defects and miscarriage. A literature article ...
-
10 OVERDOSAGERHOFADE cream is not for oral use. If oral ingestion occurs, seek medical advice. Monitor patient closely and administer appropriate supportive measures as necessary. Accidental ingestion of ...
-
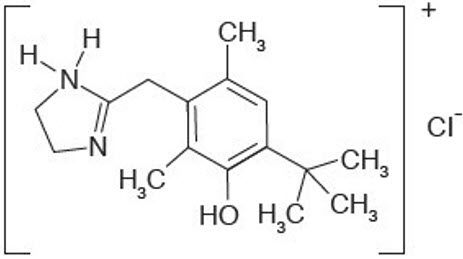
11 DESCRIPTIONRHOFADE® (oxymetazoline hydrochloride) cream, 1% contains oxymetazoline hydrochloride, an alpha1A adrenoceptor agonist. RHOFADE is a white to off-white cream. It has a chemical name of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Oxymetazoline is an alpha1A adrenoceptor agonist. Oxymetazoline acts as a vasoconstrictor. 12.2 Pharmacodynamics - The pharmacodynamics of RHOFADE cream has not been ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Oxymetazoline hydrochloride was not associated with an increased incidence of neoplastic or proliferative changes in transgenic mice ...
-
14 CLINICAL STUDIESRHOFADE cream was evaluated for the treatment of persistent erythema associated with rosacea in two identical, randomized, double-blind, vehicle-controlled, parallel-group clinical trials. The ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGRHOFADE® (oxymetazoline hydrochloride) cream, 1%, is a white to off-white cream. The product is available in a laminated tube in the following packaging configuration, each with a child-resistant ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Important Administration Instructions - Advise patients of the ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Mayne Pharma - Raleigh, NC 27609 - RHOFADE and its design are registered trademarks of Mayne Pharma, LLC - Patented. U.S. Patent Numbers: U.S. 7,812,049; U.S. 8,420,688; U.S ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - RHOFADE® (roe' fayd) (oxymetazoline hydrochloride) cream - Important: RHOFADE cream is for skin (topical) use on the face only. Do not use RHOFADE cream in your eyes ...
-
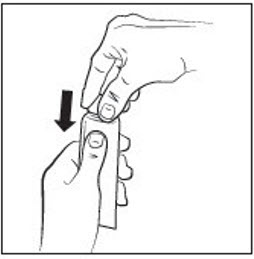
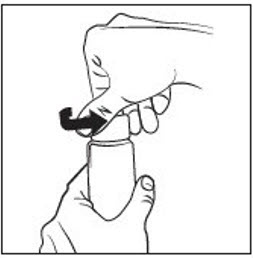
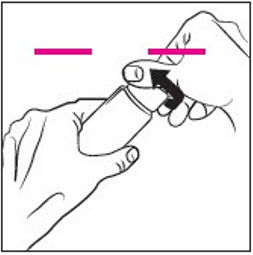
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - RHOFADE® (roe' fayd) (oxymetazoline hydrochloride) cream - Tube - Important: RHOFADE cream is for skin (topical) use on the face only. Do not use RHOFADE cream in ...
-
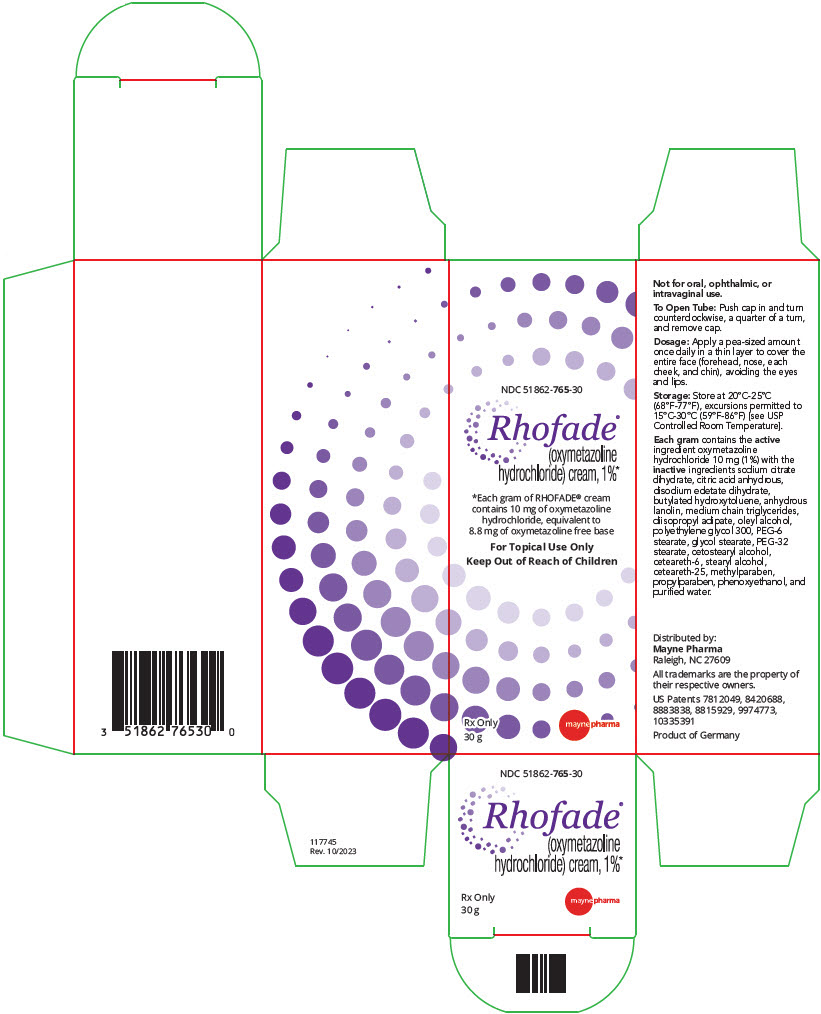
PRINCIPAL DISPLAY PANEL - 30 g Tube CartonNDC 51862-765-30 - Rhofade® (oxymetazoline - hydrochloride) cream, 1%* *Each gram of RHOFADE® cream - contains 10 mg of oxymetazoline - hydrochloride, equivalent to - 8.8 mg of oxymetazoline free ...
-
INGREDIENTS AND APPEARANCEProduct Information