Label: APHEXDA- motixafortide injection, powder, lyophilized, for solution
- NDC Code(s): 82737-073-01
- Packager: BioLineRx USA Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use APHEXDA safely and effectively. See full prescribing information for APHEXDA ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAPHEXDA is indicated in combination with filgrastim (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with ...
-
2 DOSING AND ADMINISTRATION2.1 Important Dosing Information - Premedicate all patients before each dose of APHEXDA to reduce the risk of hypersensitivity and injection site reactions: Administer ...
-
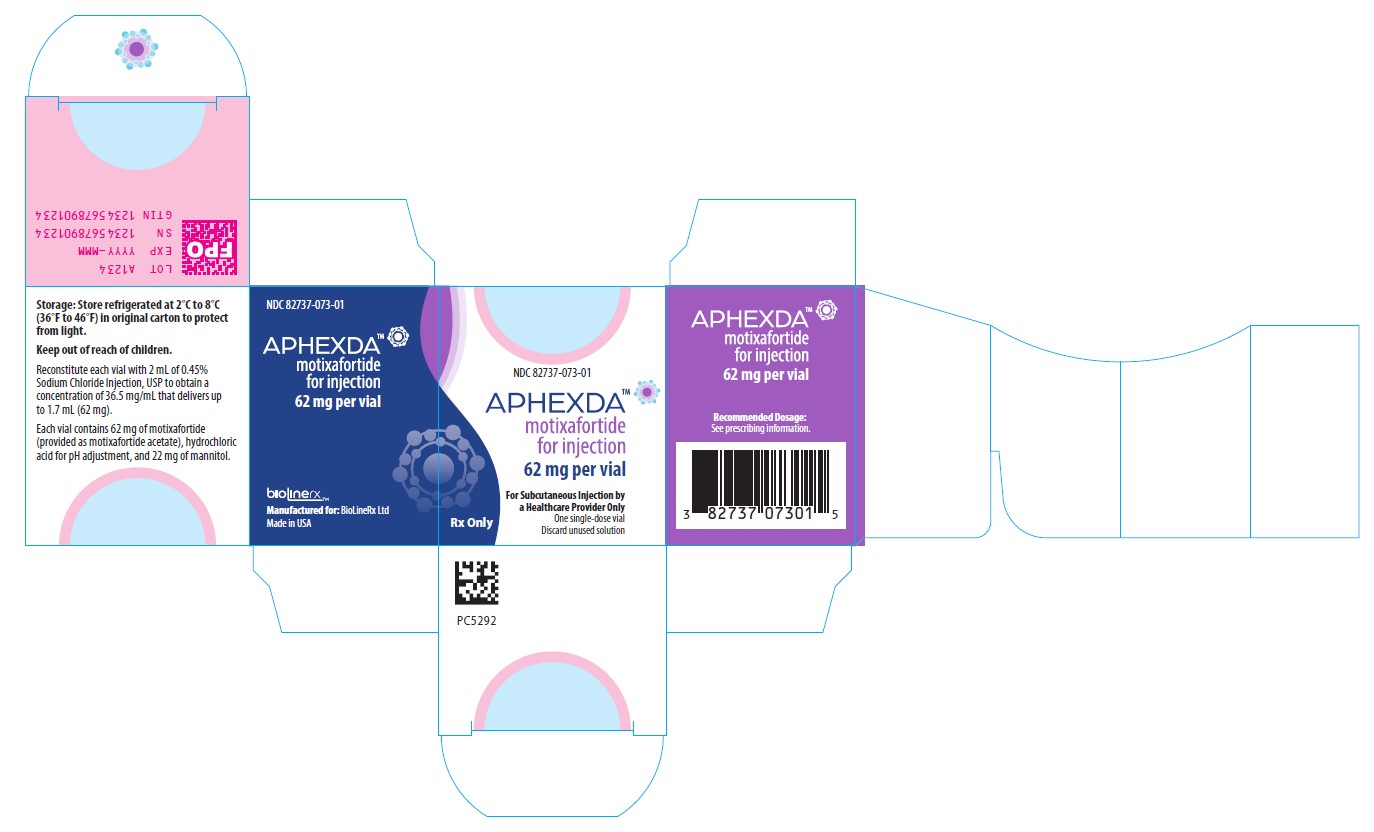
3 DOSAGE FORMS AND STRENGTHSFor injection: 62 mg as a white to off-white lyophilized powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSAPHEXDA is contraindicated in patients with a history of serious hypersensitivity reactions to motixafortide - [see - Warnings and Precautions (5.1)] .
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylactic Shock and Hypersensitivity Reactions - Anaphylactic shock occurred in 0.7% of APHEXDA-treated patients in clinical studies (n=407). The time to anaphylactic shock was between ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in other sections of the labeling: Anaphylactic Shock and Hypersensitivity Reactions [see - Warnings and Precautions (5.1) ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, APHEXDA can cause fetal harm when administered to a pregnant woman [see - Clinical Pharmacology (12.1)]. There are no ...
-
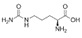
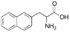
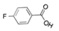
11 DESCRIPTIONAPHEXDA for injection contains motixafortide, which is a hematopoietic stem cell mobilizer. The chemical name of the synthetic motixafortide acetate, the active pharmaceutical ingredient is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Motixafortide is an inhibitor of the C-X-C Motif Chemokine Receptor 4 (CXCR4) and blocks the binding of its cognate ligand, stromal-derived factor-1α (SDF-1α)/C-X-C ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies with motixafortide have not been conducted. Motixafortide was not genotoxic in an in vitro bacterial mutation ...
-
14 CLINICAL STUDIESThe efficacy of APHEXDA in combination with filgrastim was evaluated in the GENESIS study (NCT 03246529). In this randomized, double-blind, placebo-controlled study, 122 patients with multiple ...
-
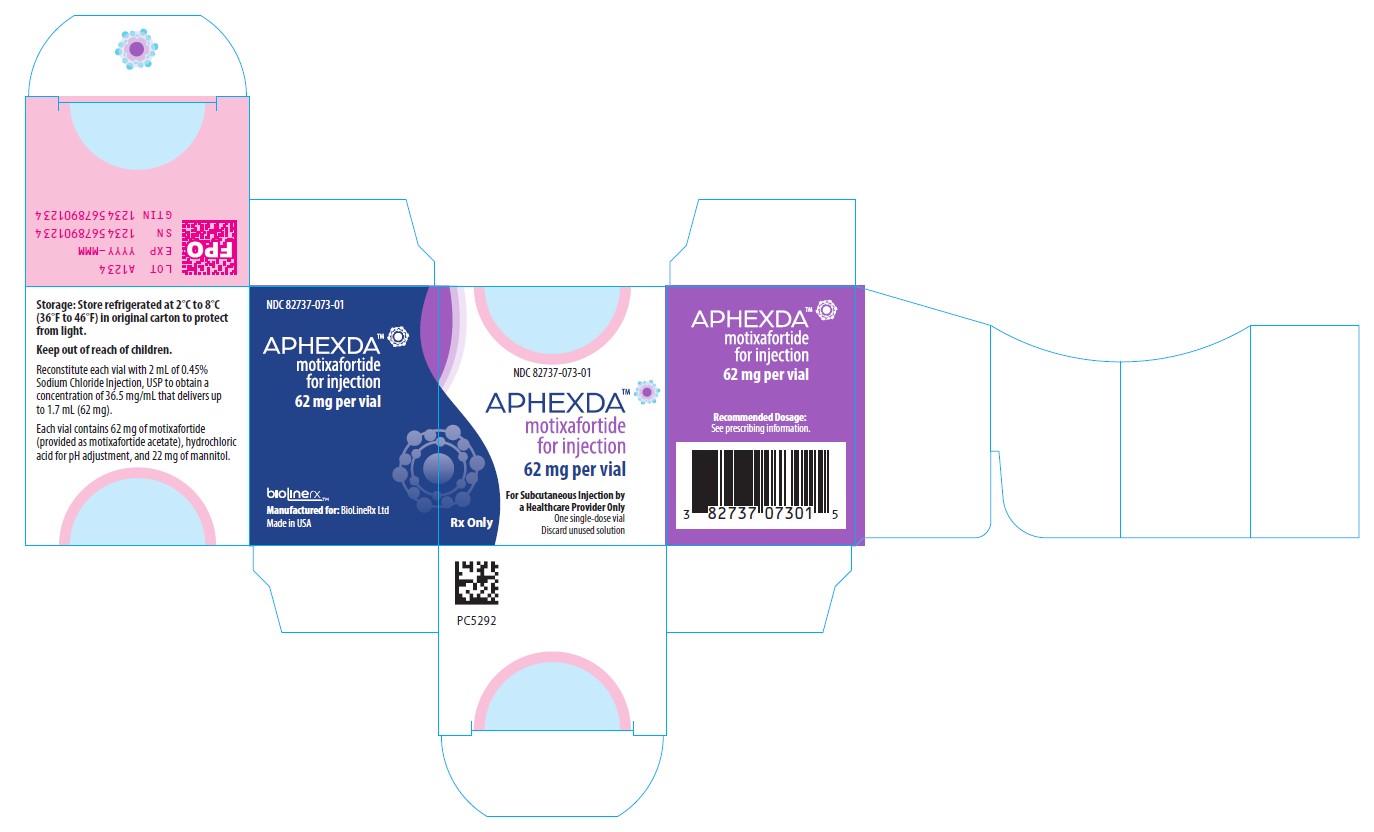
16 HOW SUPPLIED/STORAGE AND HANDLINGAPHEXDA (motixafortide) for injection is supplied as a white to off-white lyophilized powder in a single-dose vial for reconstitution. Each vial delivers 62 mg motixafortide free base. NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients of the risk of anaphylactic and hypersensitivity reactions (such as pruritus, flushing, urticaria, rash, vomiting, nausea and chills) during and after APHEXDA injection and to ...
-
PRINCIPAL DISPLAY PANELNDC 82737-073-01 - APHEXDA - TM - Motixafortide for injection - 62 mg per vial - For Subcutaneous Injection by a Healthcare Provider Only - One single-dose vial - Discard unused solution - Recommended Dosage ...
-
INGREDIENTS AND APPEARANCEProduct Information