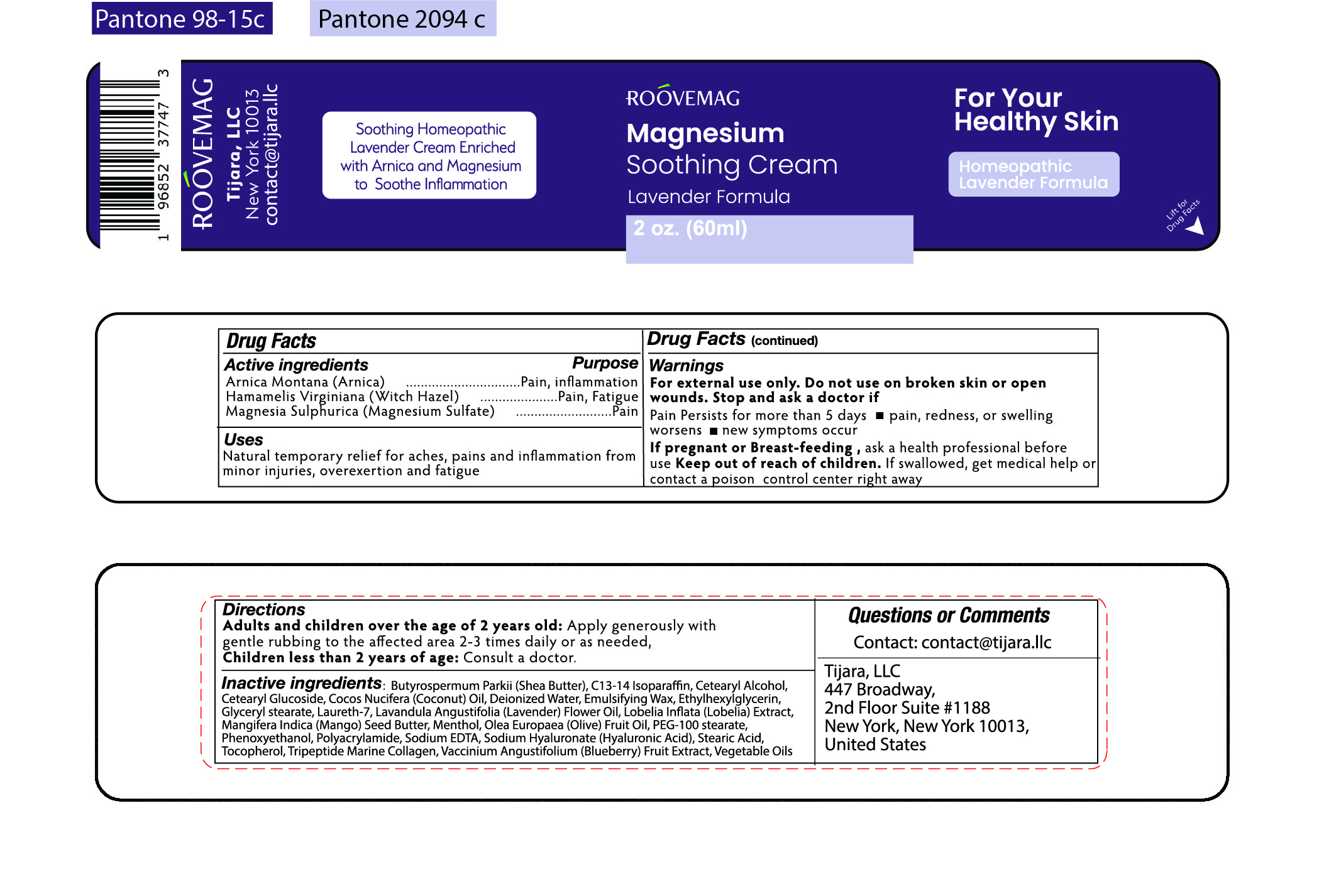
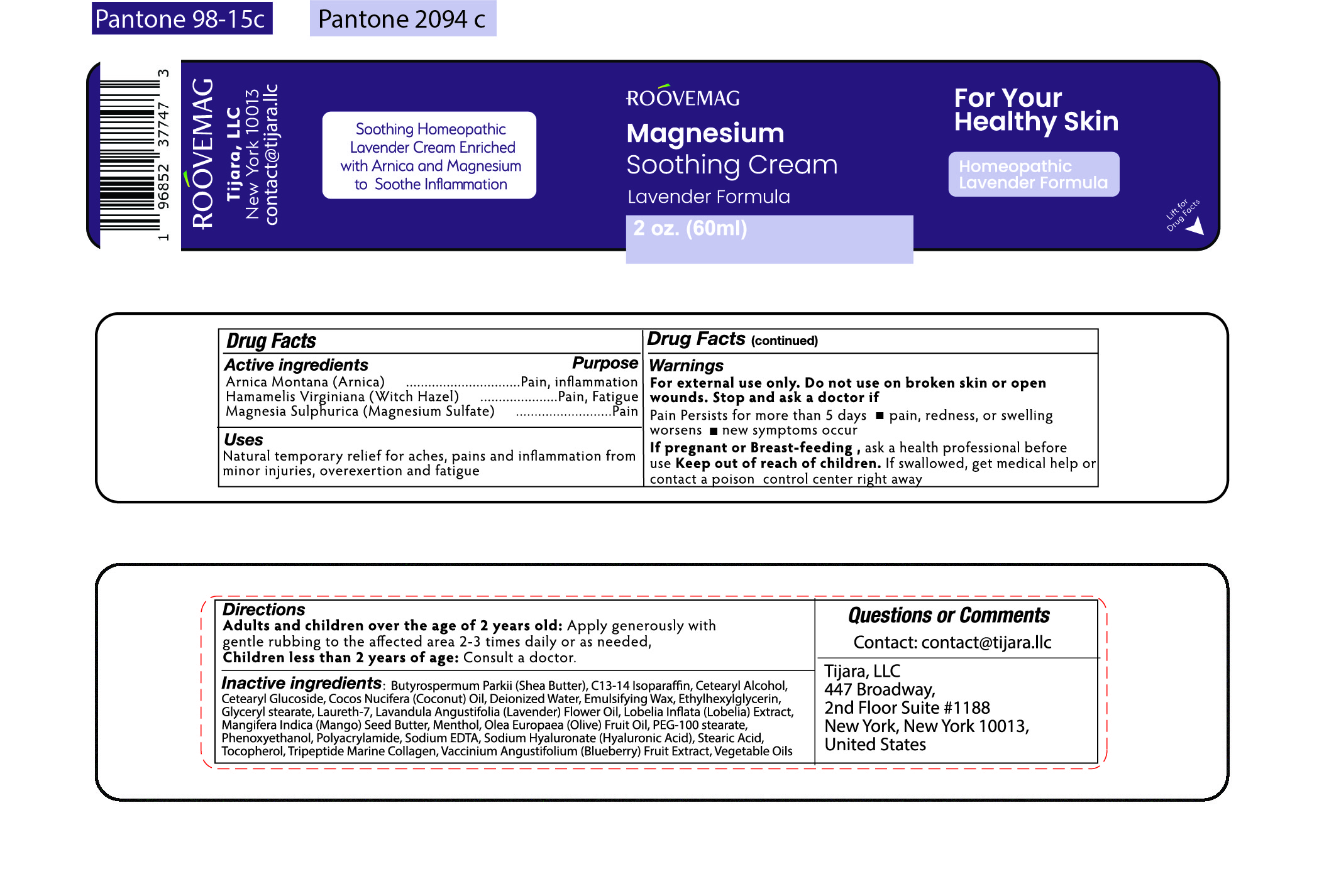
Label: ROOVEMAG MAGNESIUM SOOTHING CREAM LAVENDER- arnica, witch hazel, magnesium sulfate cream
- NDC Code(s): 76348-404-01
- Packager: RENU LABORATORIES, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 24, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
For external use only. Do not use on broken skin or open wounds.
Stop and ask a doctor if
Pain persists for more than 5 days
- pain, redness, or swelling worsens
- new symptoms occur
If pregnant or Breast-feeding, ask a health professional before use
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive Ingredients:
Butyrospermum Parkii (Shea Butter), C13-14 Isoparaffin, Cetearyl Alcohol, Cetearyl Glucoside, Cocos Nucifera (Coconut) Oil, Deionized Water, Emulsifying Wax, Ethylhexylglycerin, Glyceryl Stearate, Laureth-7, Lavandula Angustifolia (Lavender) Flower Oil,
Lobelia Inflata (Lobelia) Extract, Mangifera Indica (Mango) Seed Butter, Menthol, Olea Europaea (Olive) Fruit Oil, PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Sodium EDTA, Sodium Hyaluronate (Hyaluronic Acid), Stearic Acid, Tocopherol, Tripeptide Marine Collagen, Vaccinium Angustifolium (Blueberr) Fruit Extract, Vegetable Oils
- QUESTIONS
- STATEMENT OF IDENTITY
- PREGNANCY OR BREAST FEEDING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROOVEMAG MAGNESIUM SOOTHING CREAM LAVENDER
arnica, witch hazel, magnesium sulfate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76348-404 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SULFATE ANHYDROUS (UNII: ML30MJ2U7I) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE ANHYDROUS 0.12 g in 60 g ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) (ARNICA MONTANA FLOWER - UNII:OZ0E5Y15PZ) ARNICA MONTANA FLOWER 0.6 g in 60 g WITCH HAZEL (UNII: 101I4J0U34) (WITCH HAZEL - UNII:101I4J0U34) WITCH HAZEL 0.6 g in 60 g Inactive Ingredients Ingredient Name Strength CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM ACRYLOYLDIMETHYLTAURATE-ACRYLAMIDE COPOLYMER (1:1; 90000-150000 MPA.S) (UNII: 5F4963KLHS) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) LAURETH-7 (UNII: Z95S6G8201) OLIVE OIL (UNII: 6UYK2W1W1E) MARINE COLLAGEN, SOLUBLE (UNII: 8JC99XGU4W) MENTHOL (UNII: L7T10EIP3A) HYALURONATE SODIUM (UNII: YSE9PPT4TH) LAVENDER OIL (UNII: ZBP1YXW0H8) EDETATE SODIUM (UNII: MP1J8420LU) STEARIC ACID (UNII: 4ELV7Z65AP) PEG-100 STEARATE (UNII: YD01N1999R) TOCOPHEROL (UNII: R0ZB2556P8) BLUEBERRY (UNII: 253RUG1X1A) BUTYROSPERMUM PARKII (SHEA) BUTTER UNSAPONIFIABLES (UNII: 0C9AC7D6XU) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) COCONUT OIL (UNII: Q9L0O73W7L) WATER (UNII: 059QF0KO0R) GLYCERYL STEARATE/PEG-100 STEARATE (UNII: RD25J5V947) LOBELIA INFLATA (UNII: 9PP1T3TC5U) WHITE WAX (UNII: 7G1J5DA97F) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76348-404-01 60 g in 1 JAR; Type 0: Not a Combination Product 04/24/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/24/2023 Labeler - RENU LABORATORIES, INC. (945739449) Establishment Name Address ID/FEI Business Operations Renu Laboratories, Inc. 945739449 manufacture(76348-404)