Label: ACETAMINOPHEN tablet
- NDC Code(s): 70000-0627-1, 70000-0627-2, 70000-0627-3
- Packager: LEADER/ Cardinal Health 110, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
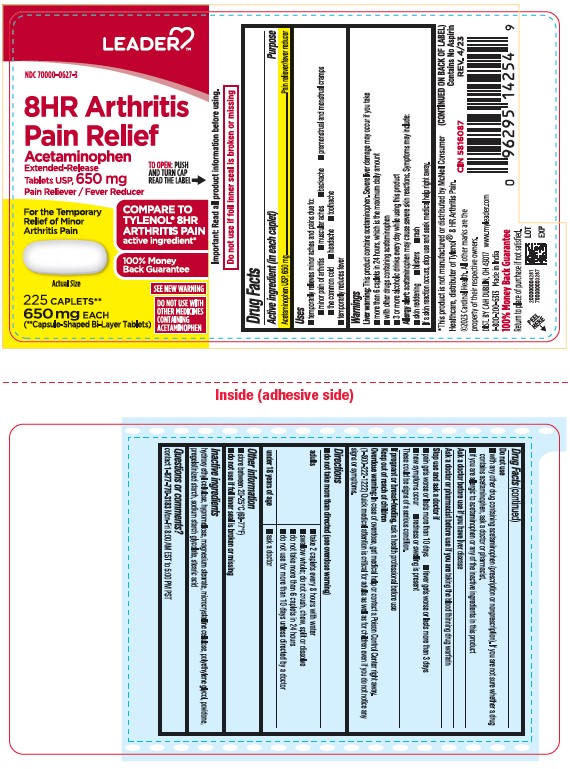
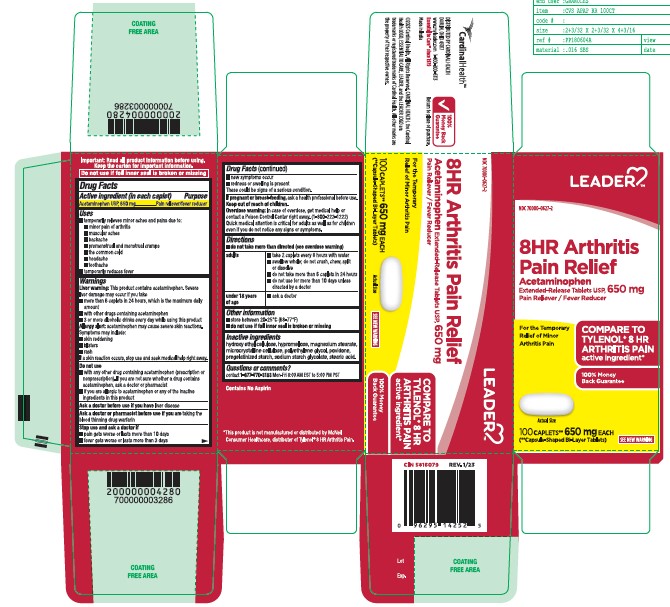
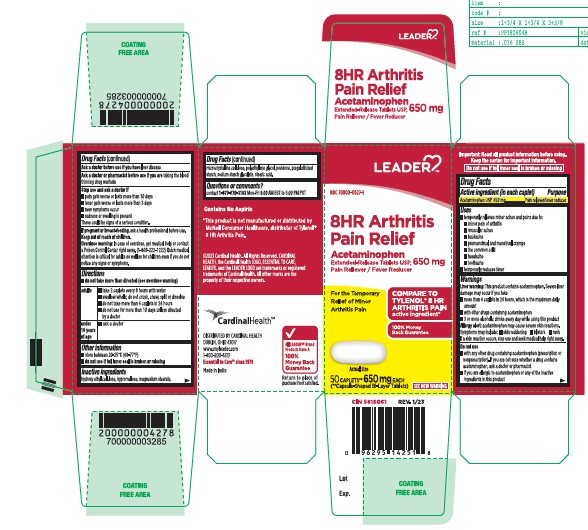
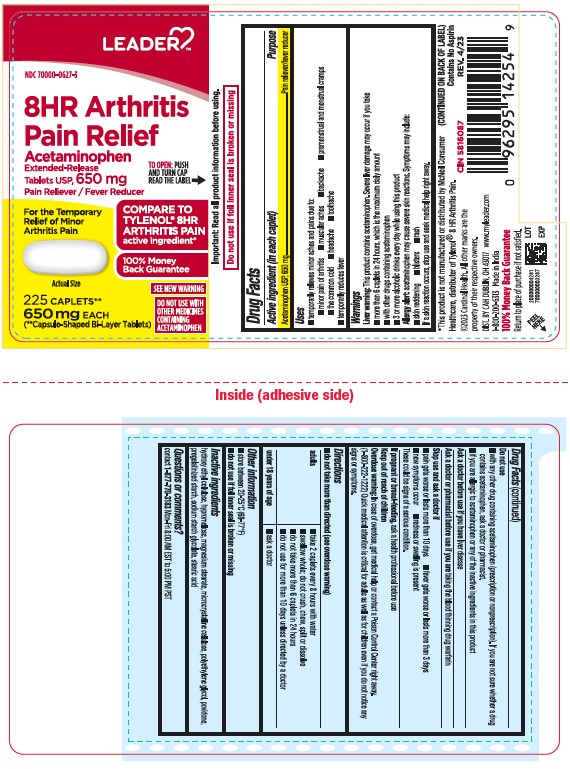
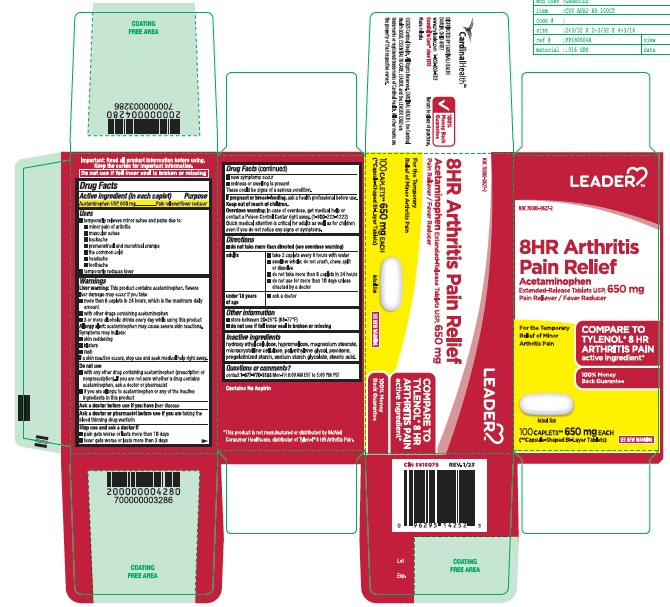
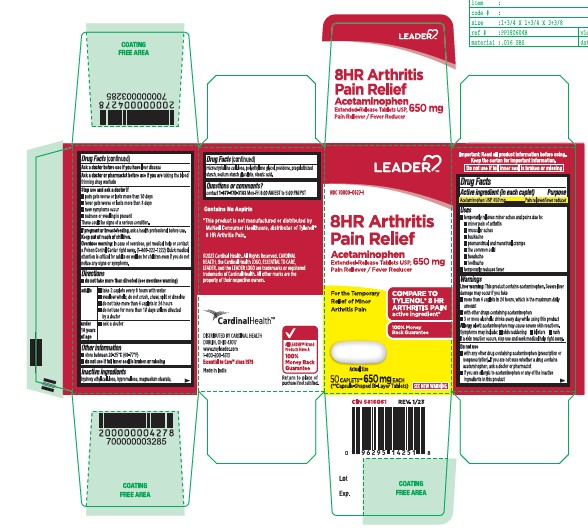
- Active ingredient (in each caplet)
- Purpose
- Uses
- Liver warning:
- Allergy alert:
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Overdose warning:
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
- PDP
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN
acetaminophen tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0627 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYDROXYETHYL CELLULOSE (140 CPS AT 5%) (UNII: 8136Y38GY5) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G650 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0627-1 50 in 1 BOTTLE; Type 0: Not a Combination Product 03/23/2023 2 NDC:70000-0627-2 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/23/2023 3 NDC:70000-0627-3 225 in 1 BOTTLE; Type 0: Not a Combination Product 03/23/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211544 03/23/2023 Labeler - LEADER/ Cardinal Health 110, Inc (063997360)