Label: GLYCOPYRROLATE injection
- NDC Code(s): 70700-902-22, 70700-902-25, 70700-903-22, 70700-903-23
- Packager: Xiromed, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 12, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
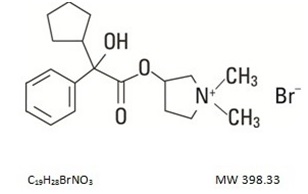
DESCRIPTIONGlycopyrrolate Injection, USP is a synthetic anticholinergic agent. Each 1 mL contains: Glycopyrrolate, USP................0.2 mg - Water for Injection, USP..........q.s. Benzyl Alcohol ...
-
CLINICAL PHARMACOLOGYGlycopyrrolate, like other anticholinergic (antimuscarinic) agents, inhibits the action of acetylcholine on structures innervated by postganglionic cholinergic nerves and on smooth muscles that ...
-
INDICATIONS AND USAGEIn Anesthesia - Glycopyrrolate injection is indicated for use as a preoperative antimuscarinic to reduce salivary, tracheobronchial, and pharyngeal secretions; to reduce the volume and free ...
-
CONTRAINDICATIONSKnown hypersensitivity to glycopyrrolate or any of its inactive ingredients. In addition, in the management of peptic ulcer patients, because of the longer duration of therapy ...
-
WARNINGSThis drug should be used with great caution, if at all, in patients with glaucoma. Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic ...
-
PRECAUTIONSGeneral - Investigate any tachycardia before giving glycopyrrolate injection since an increase in the heart rate may occur. Use with caution in patients with: coronary artery disease ...
-
ADVERSE REACTIONSAnticholinergics, including glycopyrrolate injection, can produce certain effects, most of which are extensions of their pharmacologic actions. Adverse reactions may include xerostomia (dry ...
-
OVERDOSAGETo combat peripheral anticholinergic effects, a quaternary ammonium anticholinesterase such as neostigmine methylsulfate (which does not cross the blood-brain barrier) may be given intravenously ...
-
DOSAGE AND ADMINISTRATIONNOTE: CONTAINS BENZYL ALCOHOL (see PRECAUTIONS) Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and ...
-
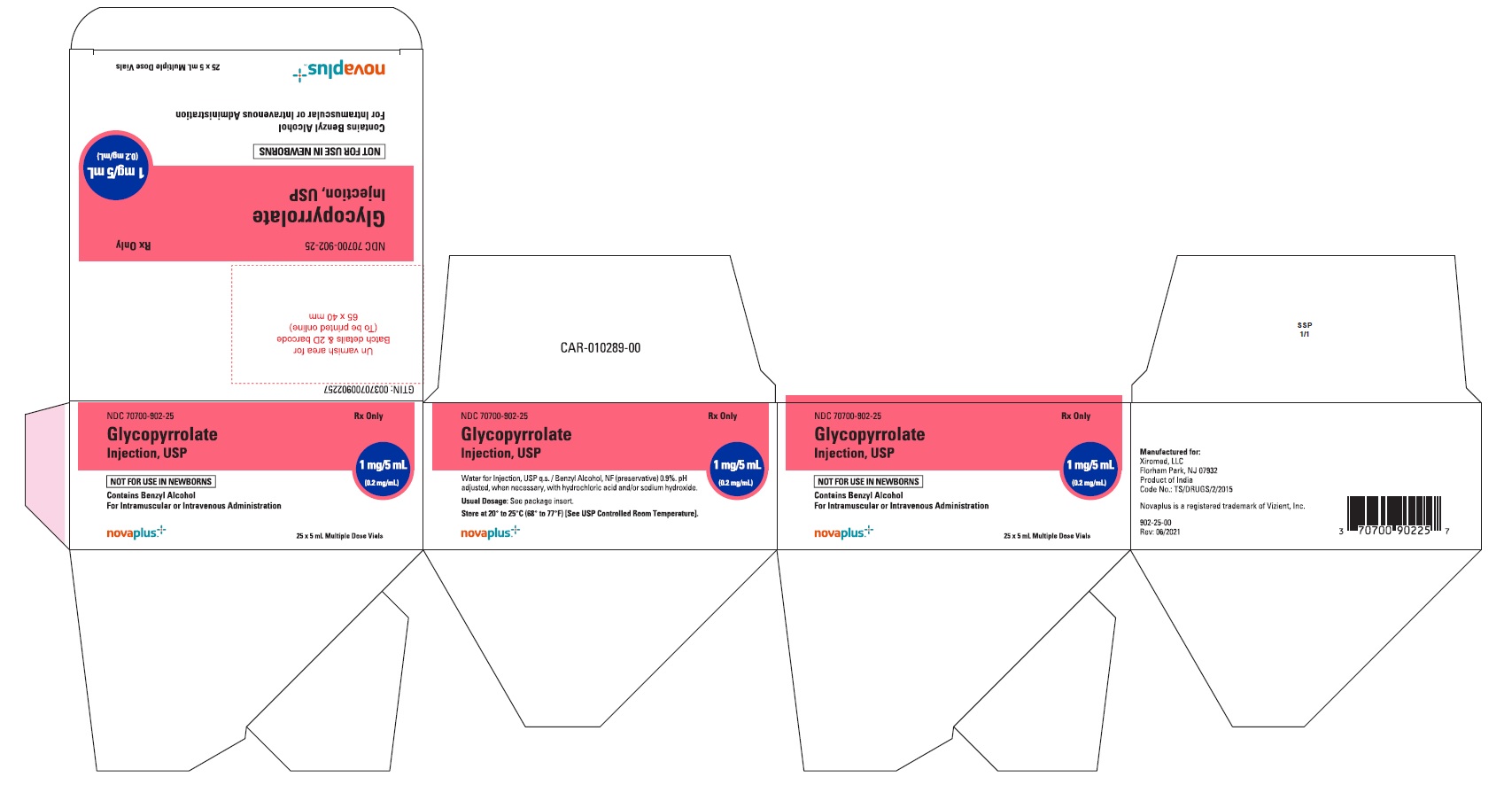
HOW SUPPLIEDGlycopyrrolate injection USP, 0.2 mg/mL, is a sterile clear, colorless solution and supplied as single and multiple dose vials available in following strengths and package sizes: 1 mg/5 ...
-
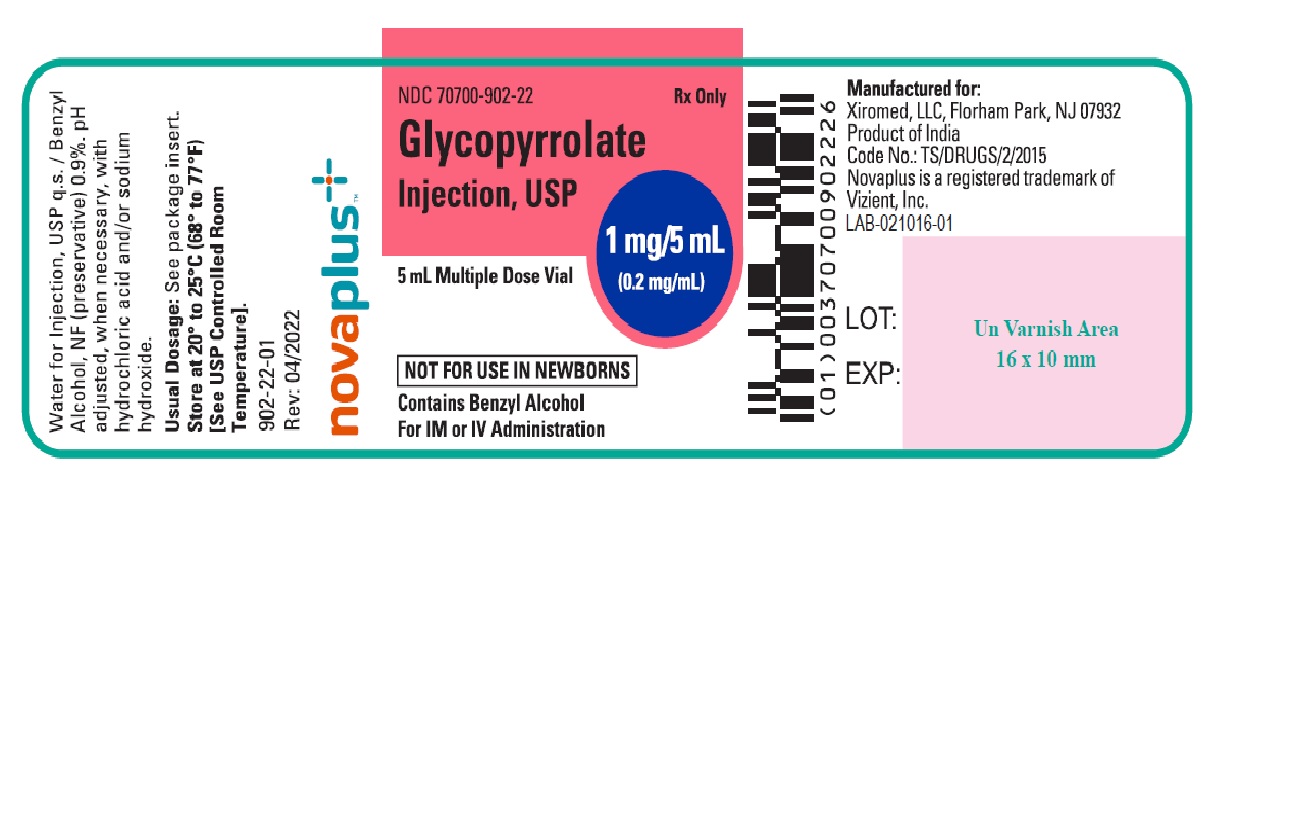
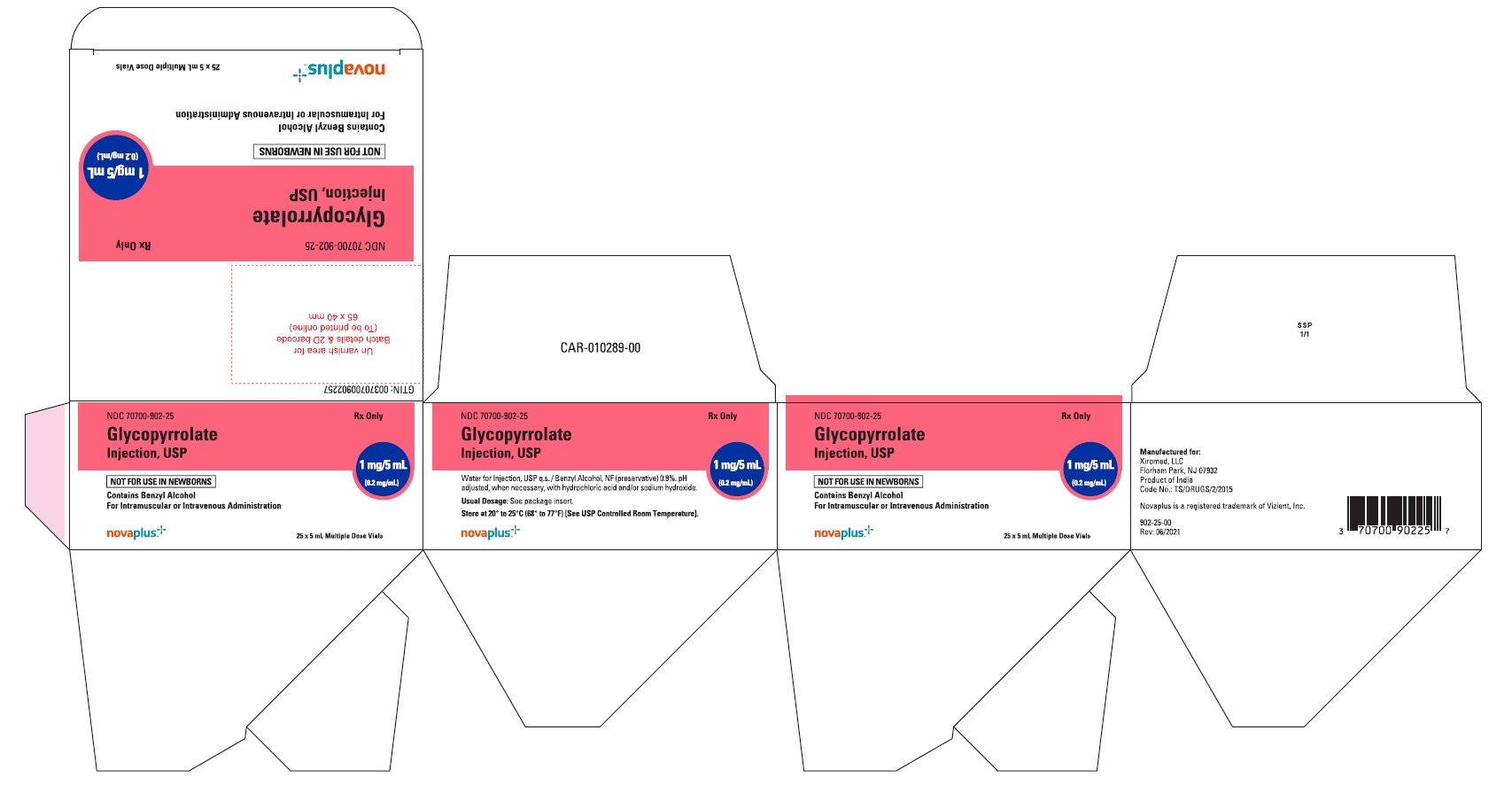
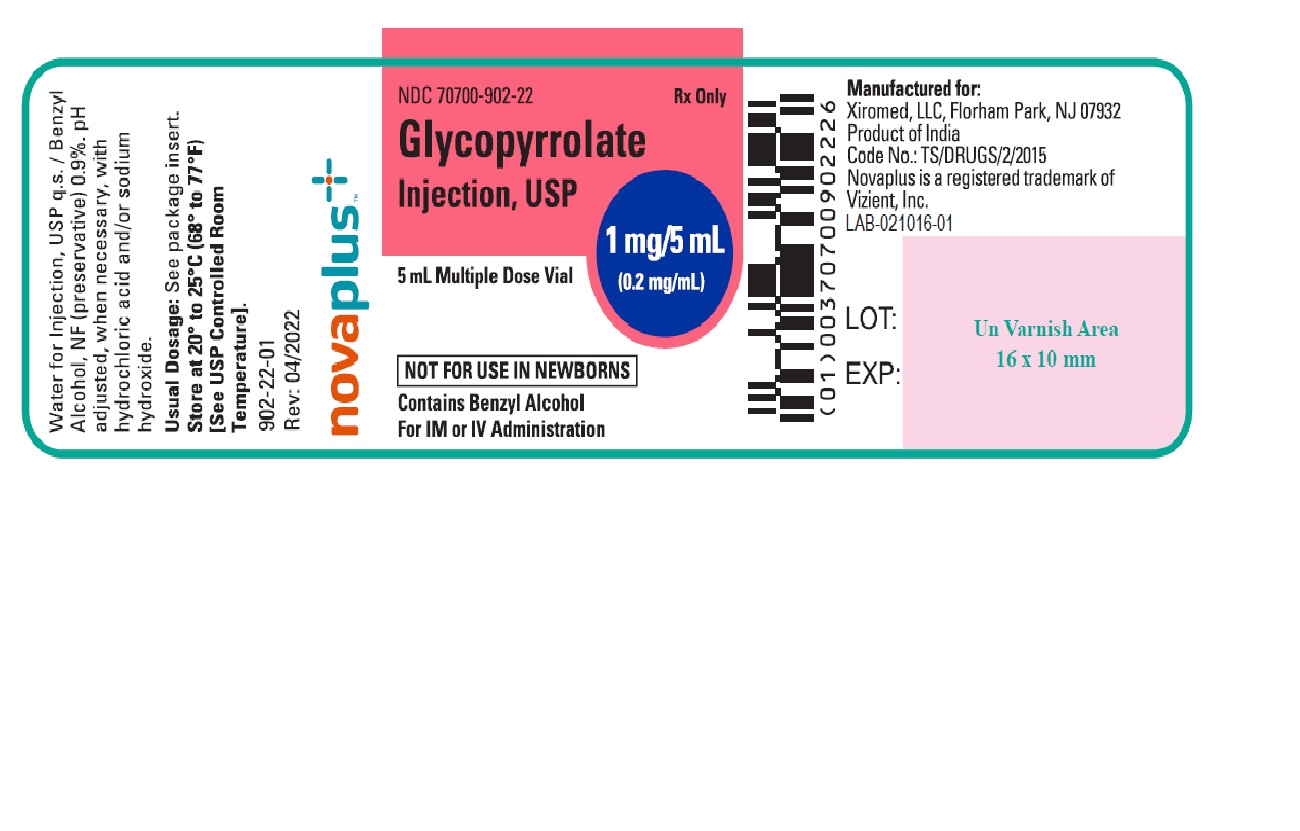
PACKAGE LABEL.PRINCIPAL DISPLAY PANELGlycopyrrolate Injection USP, 1 mg/5 mL (0.2 mg/mL) 5 mL Multiple Dose Vial Label - NDC 70700-902-22 - Glycopyrrolate Injection USP, 1 mg/5 mL (0.2 mg/mL) 25 x 5 mL Multiple Dose Vials (Carton) NDC ...
-
INGREDIENTS AND APPEARANCEProduct Information