Label: TRIFLUOPERAZINE HYDROCHLORIDE tablet, film coated
- NDC Code(s): 51079-572-01, 51079-572-20, 51079-573-01, 51079-573-20, view more
- Packager: Mylan Institutional Inc.
- This is a repackaged label.
- Source NDC Code(s): 0378-2401, 0378-2402, 0378-2405, 0378-2410
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Trifluoperazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Close -
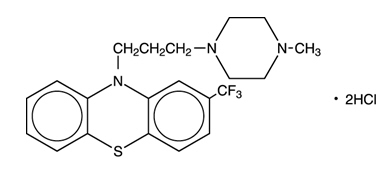
DESCRIPTIONEach film-coated tablet, for oral administration, contains trifluoperazine hydrochloride, USP equivalent to 1 mg, 2 mg, 5 mg, or 10 mg trifluoperazine. The structural formula ...
-
INDICATIONS AND USAGEFor the management of schizophrenia. Trifluoperazine hydrochloride tablets, USP are effective for the short-term treatment of generalized non-psychotic anxiety. However, trifluoperazine ...
-
CONTRAINDICATIONSA known hypersensitivity to phenothiazines, comatose or greatly depressed states due to central nervous system depressants and, in cases of existing blood dyscrasias, bone marrow depression and ...
-
WARNINGSIncreased Mortality in Elderly Patients with Dementia-Related Psychosis - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death ...
-
PRECAUTIONSGeneral - Given the likelihood that some patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be ...
-
ADVERSE REACTIONSDrowsiness, dizziness, skin reactions, rash, dry mouth, insomnia, amenorrhea, fatigue, muscular weakness, anorexia, lactation, blurred vision and neuromuscular (extrapyramidal ...
-
OVERDOSAGE(See also under - ADVERSE REACTIONS.) Symptoms - Primarily involvement of the extrapyramidal mechanism producing some of the dystonic reactions described above. Symptoms of central nervous ...
-
DOSAGE AND ADMINISTRATION-ADULTSDosage should be adjusted to the needs of the individual. The lowest effective dosage should always be used. Dosage should be increased more gradually in debilitated or emaciated patients. When ...
-
DOSAGE AND ADMINISTRATION-SCHIZOPHRENIA IN CHILDRENDosage should be adjusted to the weight of the child and severity of the symptoms. These dosages are for children, ages 6 to 12, who are hospitalized or under close supervision. Oral - The ...
-
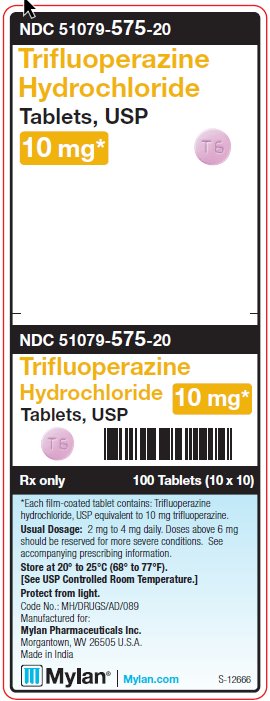
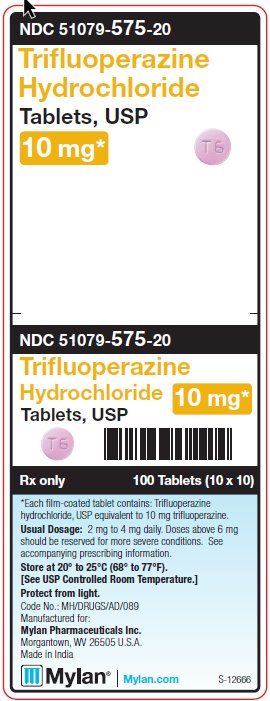
HOW SUPPLIED:Trifluoperazine Hydrochloride Tablets, USP are available containing trifluoperazine hydrochloride, USP equivalent to 1 mg, 2 mg, 5 mg or 10 mg of trifluoperazine. The 1 mg tablets are white ...
-
PRINCIPAL DISPLAY PANEL - 1 mgNDC 51079-572-20 - Trifluoperazine - Hydrochloride - Tablets, USP - 1 mg* 100 Tablets (10 x 10) *Each film-coated tablet contains: Trifluoperazine hydrochloride, USP - equivalent to 1 mg ...
-
PRINCIPAL DISPLAY PANEL - 2 mgNDC 51079-573-20 - Trifluoperazine - Hydrochloride - Tablets, USP - 2 mg* 100 Tablets (10 x 10) *Each film-coated tablet contains: Trifluoperazine hydrochloride, USP - equivalent to 2 mg ...
-
PRINCIPAL DISPLAY PANEL - 5 mgNDC 51079-574-20 - Trifluoperazine - Hydrochloride - Tablets, USP - 5 mg* 100 Tablets (10 x 10) *Each film-coated tablet contains: Trifluoperazine hydrochloride, USP - equivalent to 5 mg ...
-
PRINCIPAL DISPLAY PANEL - 10 mgNDC 51079-575-20 - Trifluoperazine - Hydrochloride - Tablets, USP - 10 mg* 100 Tablets (10 x 10) *Each film-coated tablet contains: Trifluoperazine hydrochloride, USP - equivalent to 10 ...
-
INGREDIENTS AND APPEARANCEProduct Information