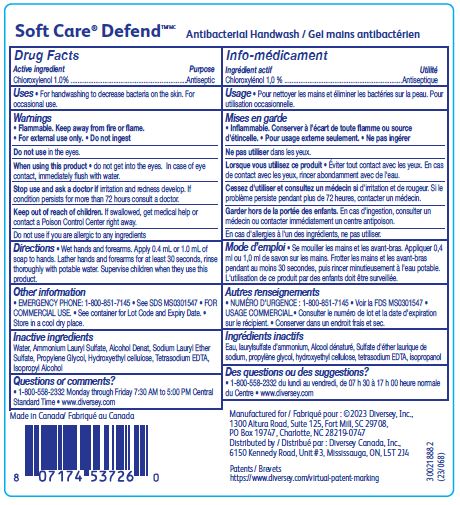
Label: SOFT CARE DEFEND ANTIBACTERIAL HANDWASH- choroxylenol solution
- NDC Code(s): 64536-8748-2, 64536-8748-3
- Packager: Diversey, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Flammable. Keep away from fire or flame.
For external use only.
Do not ingest
When using this product do not get into the eyes.
In case of eye contact, immediately flush with water.
- DOSAGE & ADMINISTRATION
- REFERENCES
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SOFT CARE DEFEND ANTIBACTERIAL HANDWASH
choroxylenol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64536-8748 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIUM LAURYL SULFATE (UNII: Q7AO2R1M0B) HYDROXYETHYL CELLULOSE (2000 MPA.S AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64536-8748-2 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/06/2016 2 NDC:64536-8748-3 1200 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/29/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 06/06/2016 Labeler - Diversey, Inc. (018240817) Establishment Name Address ID/FEI Business Operations Diversey Canada, Inc. 249266974 manufacture(64536-8748)