Label: VANCOMYCIN HYDROCHLORIDE injection, powder, lyophilized, for solution
- NDC Code(s): 63323-203-01, 63323-203-20, 63323-221-01, 63323-221-10, view more
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 15, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONVancomycin Hydrochloride - for Injection, USP - Rx only - For intravenous use. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Hydrochloride for ...
-
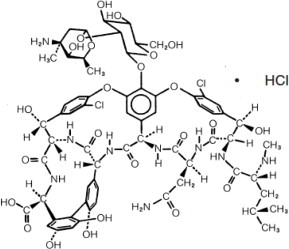
DESCRIPTION Vancomycin Hydrochloride for Injection, USP, is an off-white to buff-colored lyophilized powder, for preparing intravenous (IV) infusions, in vials each containing the equivalent of 500 mg, 750 mg ...
-
CLINICAL PHARMACOLOGY Vancomycin is poorly absorbed after oral administration. In subjects with normal kidney function, multiple intravenous dosing of 1 g of vancomycin (15 mg/kg) infused over 60 minutes produces mean ...
-
INDICATIONS AND USAGE Vancomycin Hydrochloride for Injection, USP is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (β-lactam-resistant ...
-
CONTRAINDICATIONS Vancomycin hydrochloride for injection is contraindicated in patients with known hypersensitivity to this antibiotic.
-
WARNINGS Infusion Reactions - Rapid bolus administration (e.g., over several minutes) may be associated with exaggerated hypotension, including shock and rarely cardiac arrest. Vancomycin hydrochloride ...
-
PRECAUTIONS Clinically significant serum concentrations have been reported in some patients being treated for active C. difficile-induced pseudomembranous colitis after multiple oral doses of vancomycin ...
-
ADVERSE REACTIONS To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Infusion-Related Events - During or soon after rapid ...
-
OVERDOSAGE Supportive care is advised, with maintenance of glomerular filtration. Vancomycin is poorly removed by dialysis. Hemofiltration and hemoperfusion with polysulfone resin have been reported to ...
-
DOSAGE AND ADMINISTRATION: Infusion-related events are related to both the concentration and the rate of administration of vancomycin. Concentrations of no more than 5 mg/mL and rates of no more than 10 mg/min, are ...
-
HOW SUPPLIED/STORAGE AND HANDLING Vancomycin Hydrochloride for Injection, USP, supplied as follows: Product Code - Unit of Sale - Strength - Each - 22110 - NDC 63323-221-10 - Unit of 25 - Vancomycin hydrochloride ...
-
ANIMAL PHARMACOLOGY In animal studies, hypotension and bradycardia occurred in dogs receiving an intravenous infusion of vancomycin hydrochloride 25 mg/kg, at a concentration of 25 mg/mL and an infusion rate of ...
-
REFERENCES 1. Moellering RC, Krogstad DJ, Greenblatt DJ: Vancomycin therapy in patients with impaired renal function: A nomogram for dosage. Ann Inter Med 1981; 94:343. The brand names mentioned in this ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 500 mg Vial Label NDC 63323-221-01 22110 Vancomycin Hydrochloride for Injection, USP 500 ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 500 mg Vial Tray Label NDC 63323-221-10 22110 Vancomycin Hydrochloride for Injection, USP ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 750 mg Vial Label NDC 63323-203-01 330020 Vancomycin Hydrochloride for Injection, USP 750 ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 750 mg Vial Tray Label NDC 63323-203-20 330020 Vancomycin Hydrochloride for Injection, USP ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 1 gram Vial Label NDC 63323-284-01 28420 Vancomycin Hydrochloride for Injection, USP 1 ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY – Vancomycin Hydrochloride 1 gram Vial Tray Label NDC 63323-284-20 28420 Vancomycin Hydrochloride for Injection, USP ...
-
INGREDIENTS AND APPEARANCEProduct Information