Label: MAGNESIUM SULFATE IN WATER- magnesium sulfate heptahydrate injection, solution
- NDC Code(s): 0264-4204-52, 0264-4205-52, 0264-4206-54
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONPAB® Container - For Intravenous Use Only
-
DESCRIPTIONMagnesium Sulfate in Water for Injection is a sterile, nonpyrogenic solution of magnesium sulfate heptahydrate in water for injection. May contain sulfuric acid and/or sodium hydroxide for pH ...
-
CLINICAL PHARMACOLOGYMagnesium (Mg++) is an important cofactor for enzymatic reactions and plays an important role in neurochemical transmission and muscular excitability. Magnesium prevents or controls convulsions by ...
-
INDICATIONS AND USAGEMagnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in pre-eclampsia and eclampsia, respectively. When used judiciously it effectively prevents and ...
-
CONTRAINDICATIONSIntravenous magnesium should not be given to mothers with toxemia of pregnancy during the two hours preceding delivery.
-
WARNINGSFETAL HARM: Continuous administration of magnesium sulfate beyond 5-7 days to pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus. These bone abnormalities ...
-
PRECAUTIONSBecause magnesium is removed from the body solely by the kidneys, the drug should be used with caution in patients with renal impairment. Urine output should be maintained at a level of 100 mL ...
-
ADVERSE REACTIONSThe adverse effects of parenterally administered magnesium usually are the result of magnesium intoxication. These include flushing, sweating, hypotension, depressed reflexes, flaccid paralysis ...
-
OVERDOSAGEMagnesium intoxication is manifested by a sharp drop in blood pressure and respiratory paralysis. Disappearance of the patellar reflex is a useful clinical sign to detect the onset of magnesium ...
-
DOSAGE AND ADMINISTRATIONMagnesium Sulfate in Water for Injection is intended for intravenous use only. For the management of pre-eclampsia or eclampsia, intravenous infusions of dilute solutions of magnesium (1% to 8% ...
-
HOW SUPPLIEDMagnesium Sulfate in Water for Injection is supplied in 100 mL fill and 50 mL fill PAB® containers packaged 24 per case as follows: * As the heptahydrate. † Partial fill ...
-
REFERENCESYokoyama K, Takahashi N, Yada Y. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Human Dev. 2010; 86(3):187-91. Epub 2010 Mar 12. Wedig KE, Kogan J, Schorry ...
-
SPL UNCLASSIFIED SECTIONRx only - Revised: July 2024 - PAB is a registered trademark of B. Braun Medical Inc.
-
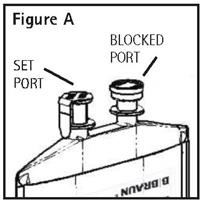
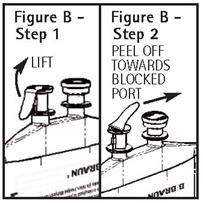
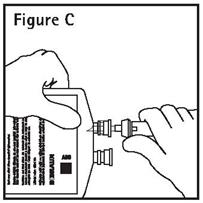
Directions For Use of PAB® Container
CAUTION: DO NOT ADD SUPPLEMENTARY MEDICATION. WHENEVER POSSIBLE USE CENTRAL ROUTE. Aseptic technique is required. Before use, perform the following checks: Read the label. Ensure solution is ...
-
SPL UNCLASSIFIED SECTIONB. Braun Medical Inc. Bethlehem, PA 18018-3524 USA - 1-800-227-2862 - Prepared in USA. API from USA or Czech Republic. Y36-003-088 LD-475-2
-
PRINCIPAL DISPLAY PANELNDC 0264-4204-52 - Magnesium Sulfate - In Water for Injection - 2 g/50 mL (40 mg/mL) (0.325 mEq Mg++/mL) 2 g Total - 50 mL PAB® Container Each 50 mL contains Magnesium Sulfate ...
-
PRINCIPAL DISPLAY PANELNDC 0264-4205-52 - Magnesium Sulfate - In Water for Injection - 4 g/50 mL (80 mg/mL) (0.65 mEq Mg++/mL) 4 g Total - 50 mL PAB® Container Each 50 mL contains Magnesium Sulfate ...
-
PRINCIPAL DISPLAY PANELNDC 0264-4206-54 - Magnesium Sulfate - In Water for Injection - 4 g/100 mL (40 mg/mL) (0.325 mEq Mg++/mL) 4 g Total - 100 mL PAB® Container Each 100 mL contains Magnesium Sulfate ...
-
INGREDIENTS AND APPEARANCEProduct Information