Label: SENNA- sennosides capsule, gelatin coated
- NDC Code(s): 69842-320-60
- Packager: CVS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
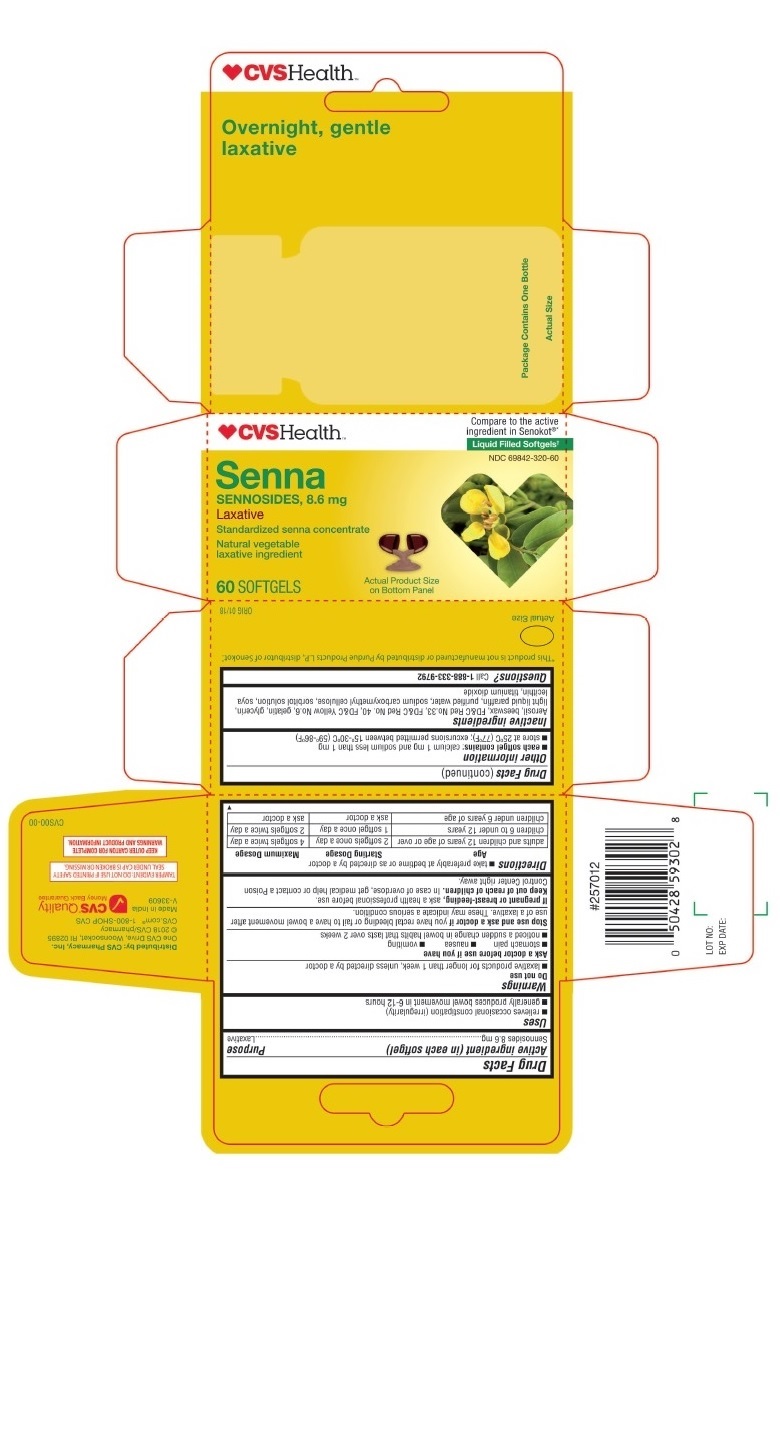
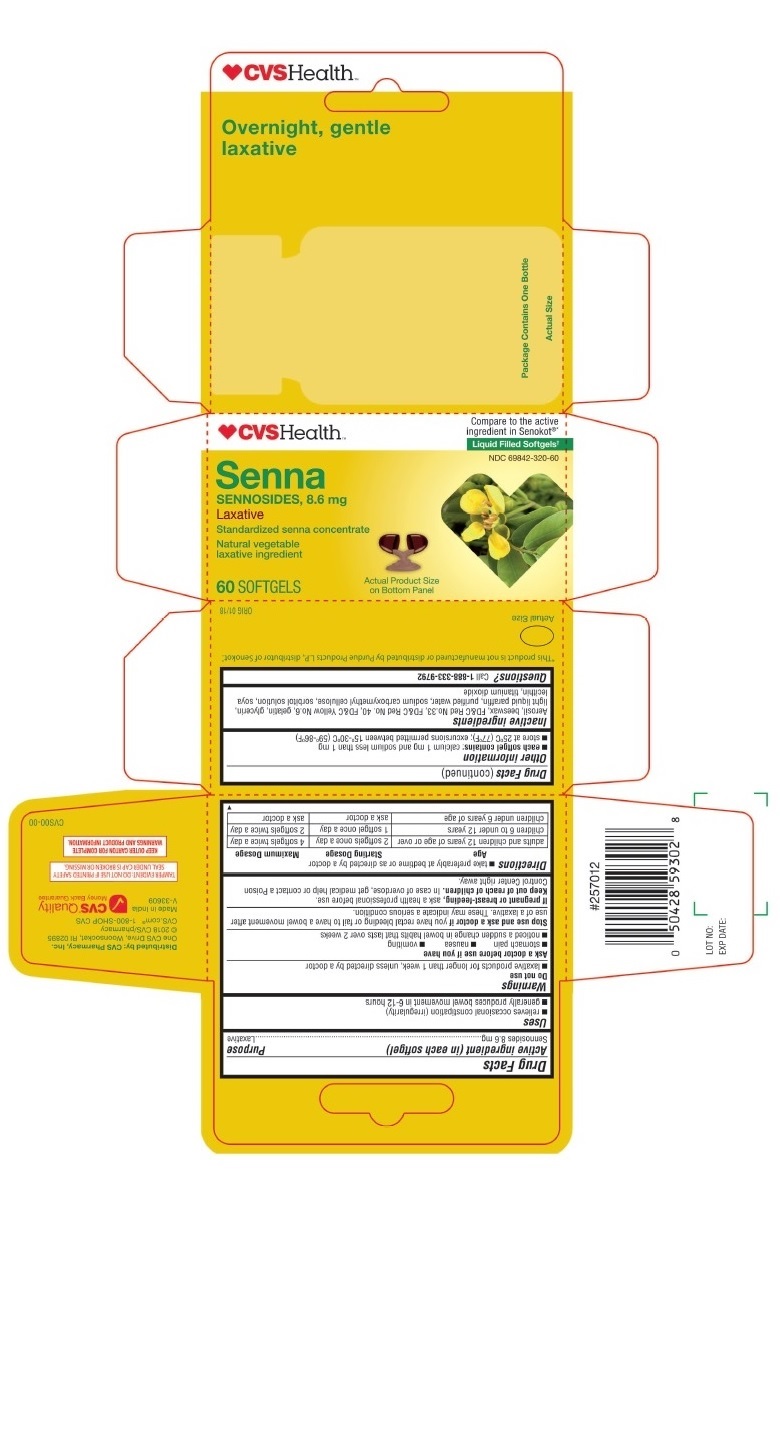
- PRINCIPAL DISPLAY PANEL - CVS SENNA 60 Softgels Bottle Carton
-
INGREDIENTS AND APPEARANCE
SENNA
sennosides capsule, gelatin coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-320 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength YELLOW WAX (UNII: 2ZA36H0S2V) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) WATER (UNII: 059QF0KO0R) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) SORBITOL (UNII: 506T60A25R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color brown (Opaque) Score no score Shape CAPSULE (SOFTGEL) Size 12mm Flavor Imprint Code 458 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-320-60 1 in 1 CARTON 03/14/2018 1 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 03/14/2018 Labeler - CVS (062312574)