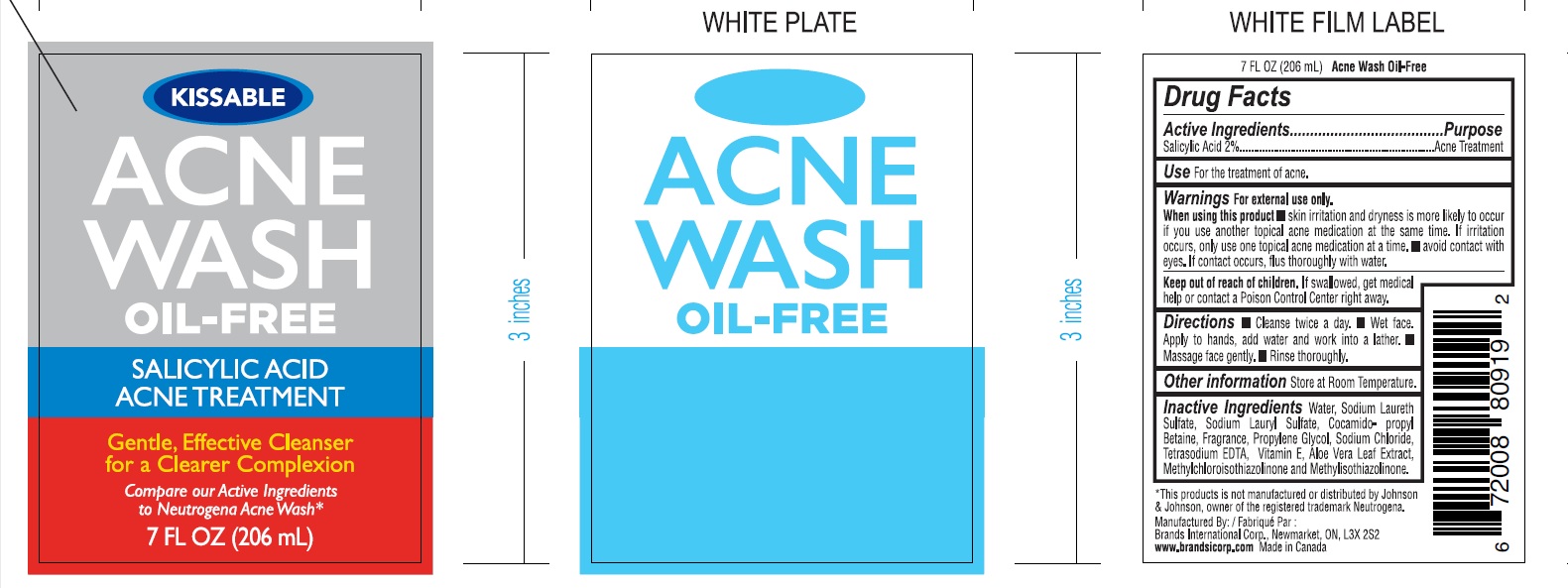
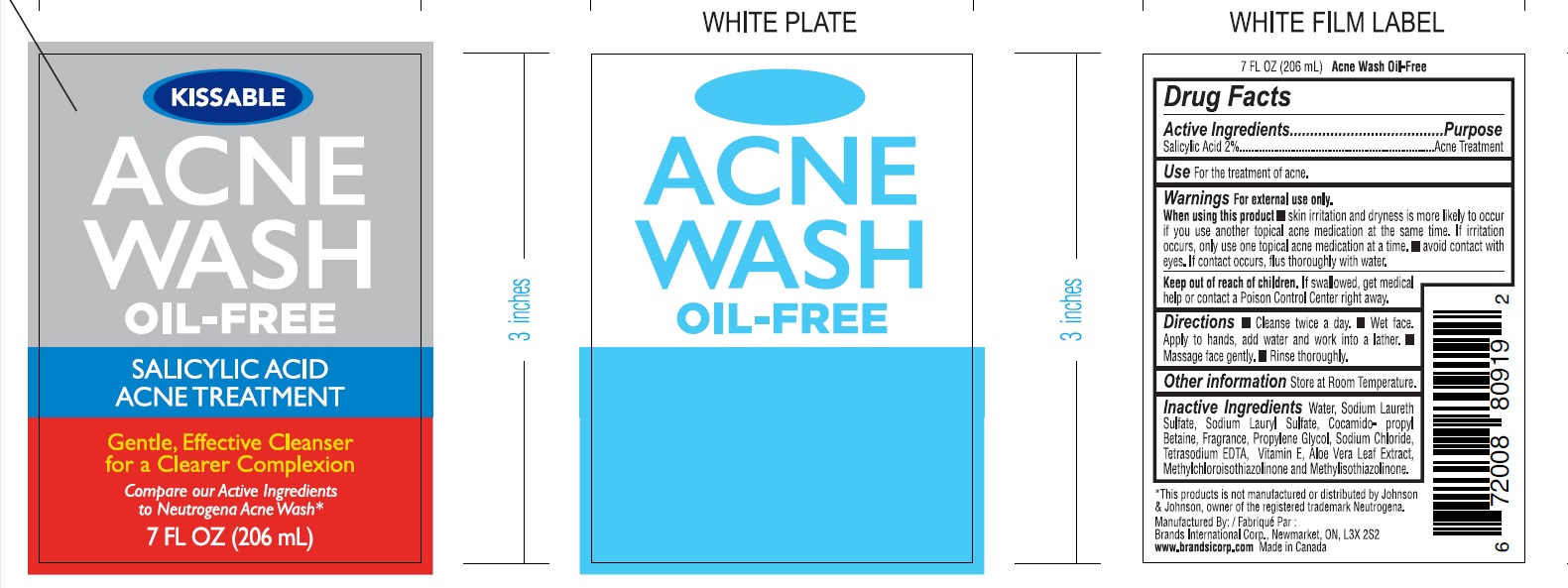
Label: ACNE WASH KISSABLE- salicylic acid gel
- NDC Code(s): 50157-012-20, 50157-012-23
- Packager: BRANDS INTERNATIONAL
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warning: for external use only.
Ask a doctor or pharmacist before use if you are using other topical acne medications at the same time or immediately following use of this product. This may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
When using this product avoid contact with eyes. If contact occurs, flush thoroughly with water.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACNE WASH KISSABLE
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50157-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) SODIUM CHLORIDE (UNII: 451W47IQ8X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50157-012-20 206 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/27/2023 2 NDC:50157-012-23 236 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/27/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 02/27/2023 Labeler - BRANDS INTERNATIONAL (243748238) Establishment Name Address ID/FEI Business Operations Brands International 243748238 manufacture(50157-012)