Label: MAGNESIUM SULFATE IN WATER injection, solution
- NDC Code(s): 83634-500-41, 83634-500-42, 83634-500-81, 83634-500-82, view more
- Packager: Avenacy Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONAVENACY - Rx only
-
DESCRIPTION
Magnesium Sulfate in Water for Injection is a sterile, nonpyrogenic solution of magnesium sulfate heptahydrate in water for injection. May contain sulfuric acid and/or sodium hydroxide for pH ...
-
CLINICAL PHARMACOLOGY
Magnesium (Mg++) is an important cofactor for enzymatic reactions and plays an important role in neurochemical transmission and muscular excitability. Magnesium prevents or controls convulsions ...
-
INDICATIONS AND USAGE
Magnesium Sulfate in Water for Injection is indicated for the prevention and control of seizures in preeclampsia and eclampsia, respectively. When used judiciously it effectively prevents and ...
-
CONTRAINDICATIONS
Intravenous magnesium should not be given to mothers with toxemia of pregnancy during the two hours preceding delivery.
-
WARNINGS
FETAL HARM: Continuous administration of magnesium sulfate beyond 5-7 days to pregnant women can lead to hypocalcemia and bone abnormalities in the developing fetus. These bone abnormalities ...
-
PRECAUTIONS
Because magnesium is removed from the body solely by the kidneys, the drug should be used with caution in patients with renal impairment. Urine output should be maintained at a level of 100 mL ...
-
ADVERSE REACTIONS
The adverse effects of parenterally administered magnesium usually are the result of magnesium intoxication. These include flushing, sweating, hypotension, depressed reflexes, flaccid paralysis ...
-
OVERDOSAGE
Magnesium intoxication is manifested by a sharp drop in blood pressure and respiratory paralysis. Disappearance of the patellar reflex is a useful clinical sign to detect the onset of magnesium ...
-
DOSAGE AND ADMINISTRATION
Magnesium Sulfate in Water for Injection is intended for intravenous use only. For the management of pre-eclampsia or eclampsia, intravenous infusions of dilute solutions of magnesium (1% to 8% ...
-
HOW SUPPLIED
Magnesium Sulfate in Water for Injection is supplied in single-dose flexible plastic containers as follows: * Partial fill container 50 mL volume in 100 mL container. ** As the ...
-
REFERENCES
Yokoyama K, Takahashi N, Yada Y. Prolonged maternal magnesium administration and bone metabolism in neonates. Early Human Dev. 2010; 86(3):187-91. Epub 2010 Mar 12. Wedig KE, Kogan J, Schorry EK ...
-
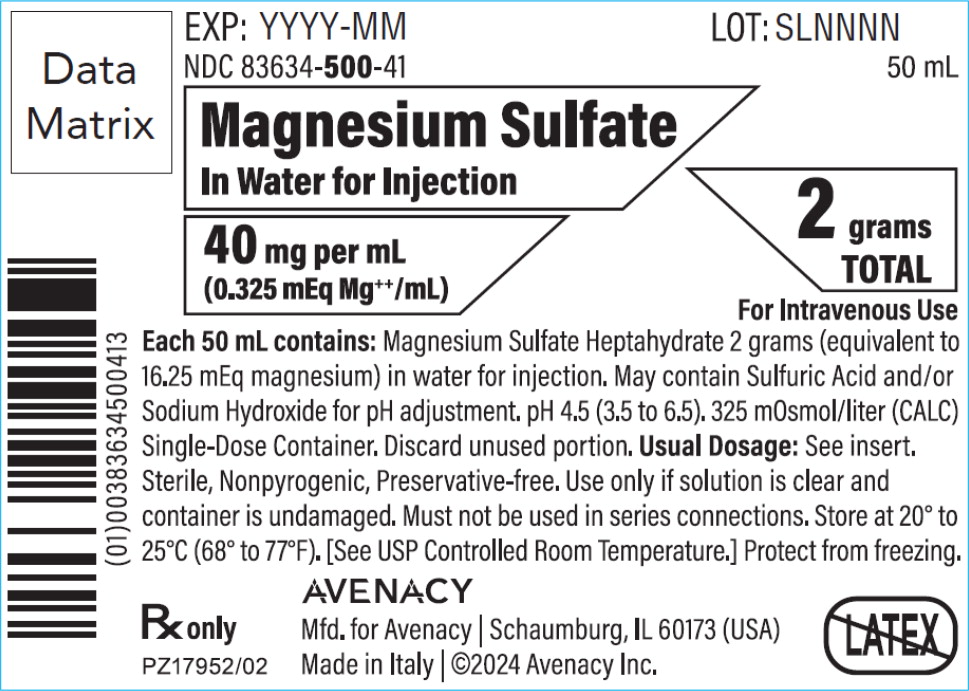
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BAG - NDC 83634-500-41 - 50 mL - Magnesium Sulfate In Water for Injection - 40 mg per mL (0.325 mEq Mg++/mL) 2 grams TOTAL - For Intravenous Use - Rx ...
-
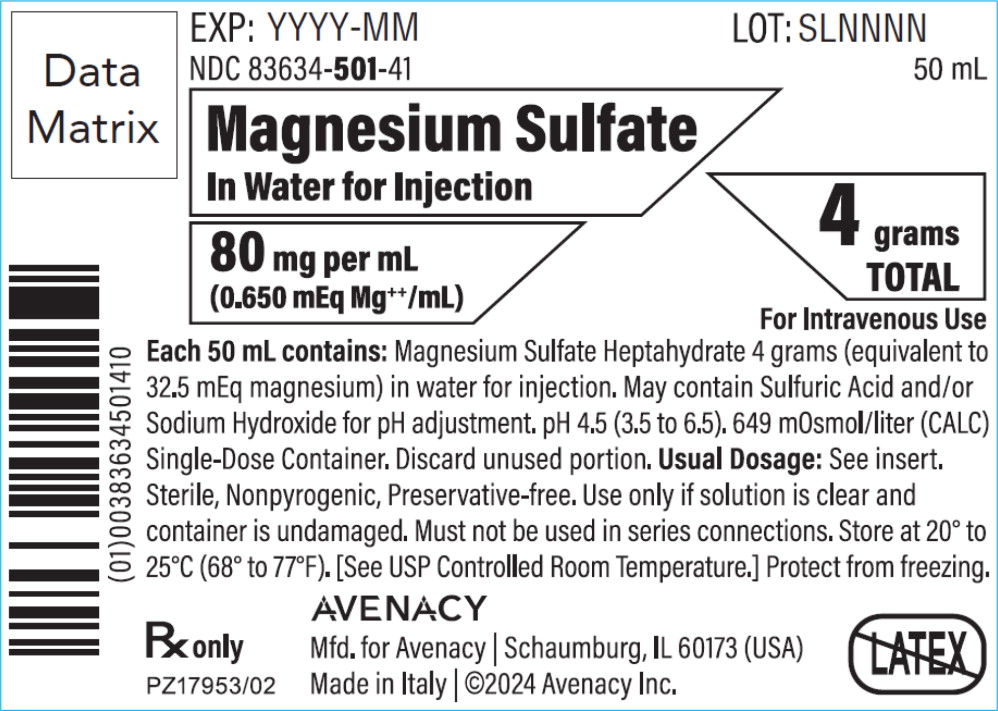
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BAG - NDC 83634-501-41 - 50 mL - Magnesium Sulfate In Water for Injection - 80 mg per mL (0.650 mEq Mg++/mL) 4 grams TOTAL - For Intravenous Use - Rx ...
-
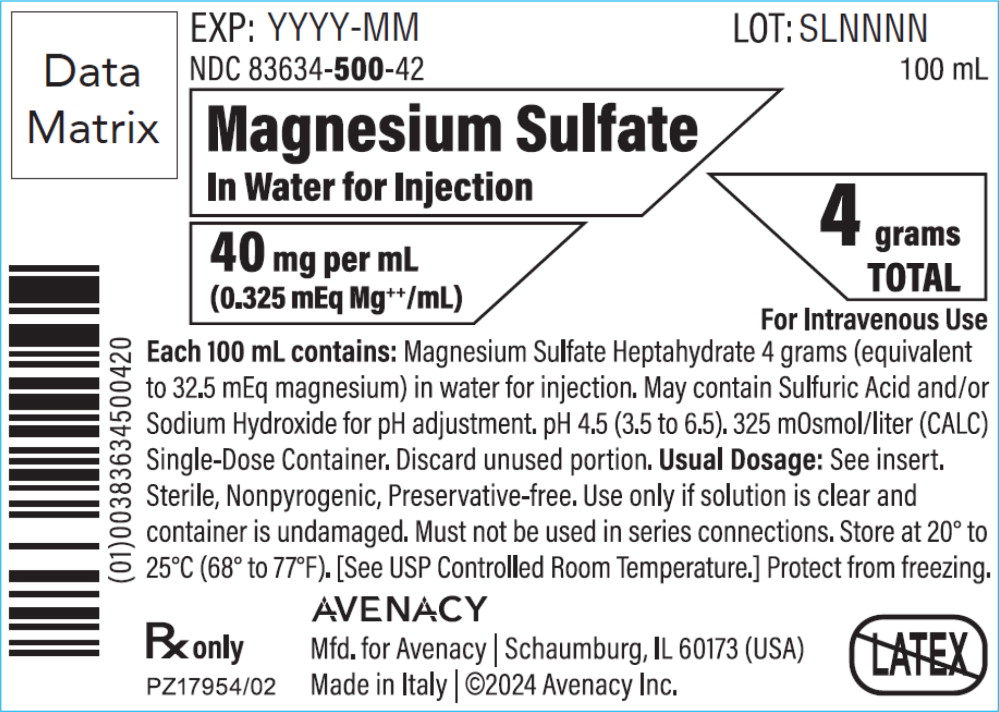
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – BAG - NDC 83634-500-42 - 100 mL - Magnesium Sulfate In Water for Injection - 40 mg per mL (0.325 mEq Mg++/mL) 4 grams TOTAL - For Intravenous Use - Rx ...
-
INGREDIENTS AND APPEARANCEProduct Information