Label: ULTRAMICROSIZE GRISEOFULVIN tablet, coated
- NDC Code(s): 0781-5827-01, 0781-5827-05, 0781-5828-01, 0781-5828-05
- Packager: Sandoz Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
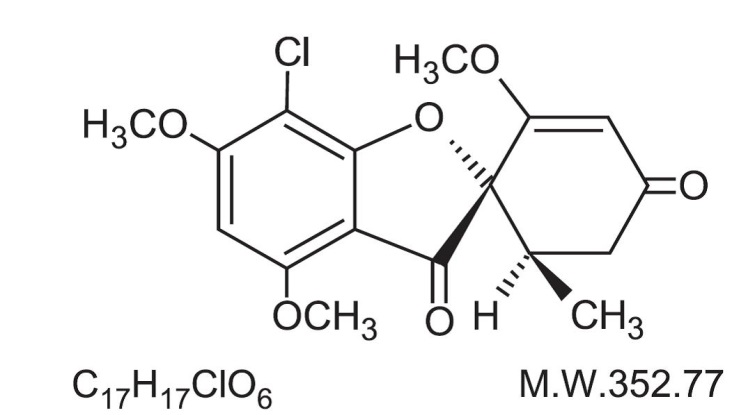
DESCRIPTIONUltramicrosize griseofulvin tablets, USP contain ultramicrosize crystals of griseofulvin, an antibiotic derived from a species of Penicillium. The chemical name of griseofulvin, USP is ...
- CLINICAL PHARMACOLOGY
-
MicrobiologyGriseofulvin is fungistatic with in vitro activity against various species of Microsporum, Epidermophyton and Trichophyton. It has no effect on bacteria or other genera of fungi.
-
PharmacokineticsFollowing oral administration, griseofulvin is deposited in the keratin precursor cells and has a greater affinity for diseased tissue. The drug is tightly bound to the new keratin which becomes ...
-
INDICATIONS AND USAGE Ultramicrosize griseofulvin tablets are indicated for the treatment of the following ringworm infections; tinea corporis (ringworm of the body), tinea pedis (athlete’s foot), tinea cruris ...
-
CONTRAINDICATIONSTwo cases of conjoined twins have been reported since 1977 in patients taking griseofulvin during the first trimester of pregnancy. Griseofulvin should not be prescribed to pregnant patients. If ...
-
WARNINGSProphylactic Usage - Safety and efficacy of griseofulvin for prophylaxis of fungal infections have not been established. Serious Skin Reactions - Severe skin reactions (e.g. Stevens-Johnson ...
-
PRECAUTIONSPatients on prolonged therapy with any potent medication should be under close observation. Periodic monitoring of organ system function, including renal, hepatic and hematopoietic, should be ...
-
ADVERSE REACTIONSThere have been post-marketing reports of severe skin and hepatic adverse events associated with griseofulvin use (see WARNINGS section). When adverse reactions occur, they are most commonly of ...
-
DOSAGE AND ADMINISTRATIONAccurate diagnosis of infecting organism is essential. Identification should be made either by direct microscopic examination of a mounting of infected tissue in a solution of potassium hydroxide ...
-
HOW SUPPLIEDUltramicrosize griseofulvin tablets, USP are available as follows: 125 mg, are yellow colored, oval shaped, film coated biconvex tablets debossed with ‘I127’ on one side and scored on other ...
-
Principal Display PanelNDC 0781-5827-01 - Ultramicrosize Griseofulvin Tablets, USP - 125 mg - Rx only - 100 Tablets
-
Principal Display PanelNDC 0781-5828-01 - Ultramicrosize Griseofulvin Tablets, USP - 250 mg - Rx only - 100 Tablets
-
INGREDIENTS AND APPEARANCEProduct Information