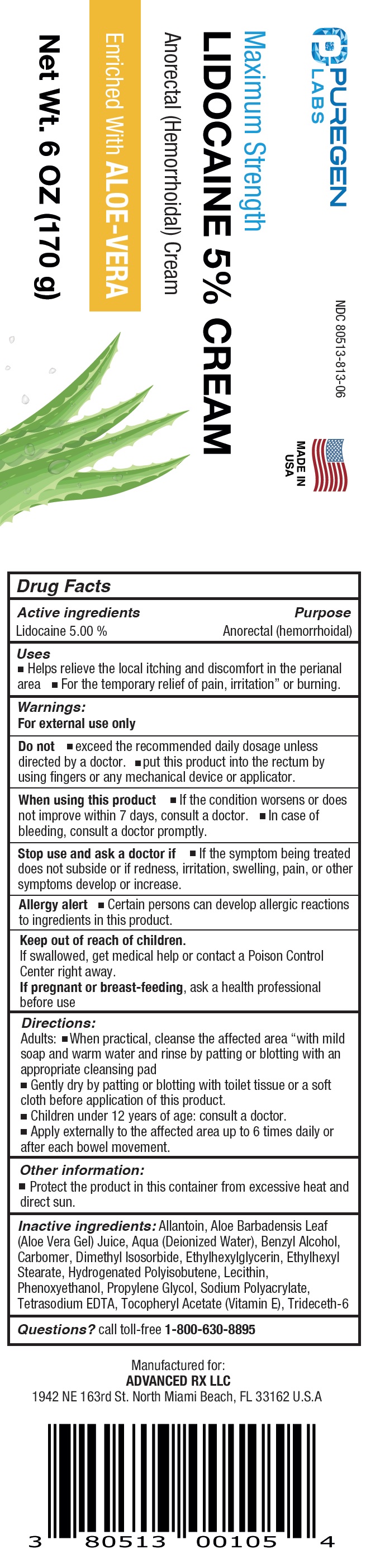
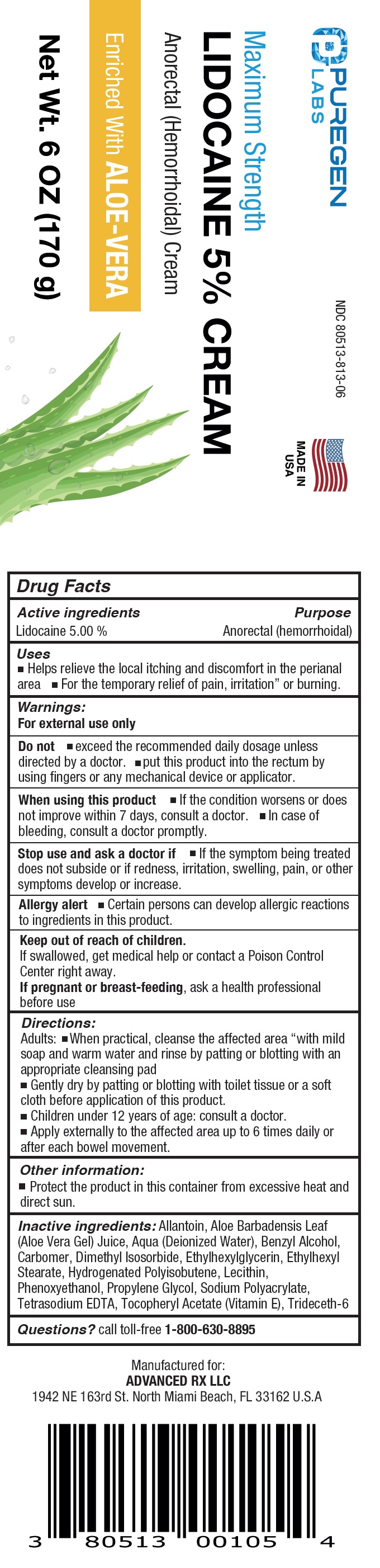
Label: PUREGEN LABS MAXIMUM STRENGTH LIDOCAINE- lidocaine cream
- NDC Code(s): 80513-813-06
- Packager: Advanced Rx LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
-
Warnings:
For external use only
Do not
- exceed the recommended daily dosage unless directed by a doctor.
- put this product into the rectum by using fingers or any mechanical device or applicator.
When using this product
- If the condition worsens or does not improve within 7 days, consult a doctor.
- In case of bleeding, consult a doctor promptly.
Stop use and ask a doctor if
- If the symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase.
Allergy alert
- Certain persons can develop allergic reactions to ingredients in this product.
-
Directions:
Adults:
- When practical, cleanse the affected area “with mild soap and warm water and rinse by patting or blotting with an appropriate cleansing pad
- Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Children under 12 years of age: consult a doctor.
- Apply externally to the affected area up to 6 times daily or after each bowel movement.
- Other information:
-
Inactive ingredients:
Allantoin, Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionized Water), Benzyl Alcohol, Carbomer, Dimethyl Isosorbide, Ethylhexylglycerin, Ethylhexyl Stearate, Hydrogenated Polyisobutene, Lecithin, Phenoxyethanol, Propylene Glycol, Sodium Polyacrylate, Tetrasodium EDTA, Tocopheryl Acetate (Vitamin E), Trideceth-6
- Questions?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
PUREGEN LABS MAXIMUM STRENGTH LIDOCAINE
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80513-813 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE SODIUM (UNII: MP1J8420LU) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIDECETH-6 (UNII: 3T5PCR2H0C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80513-813-06 170 g in 1 TUBE; Type 0: Not a Combination Product 04/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 04/22/2024 Labeler - Advanced Rx LLC (042795108)