Label: FOLIC ACID tablet
- NDC Code(s): 72162-2413-0, 72162-2413-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 72241-050
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
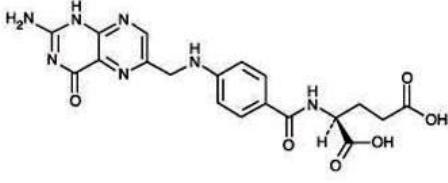
DESCRIPTIONFolic acid, USP, N-[p-[[(2-amino-4- hydroxy-6-pteridinyl methyl]- amino] benzoyl]-Lglutamic acid, is a B complex vitamin containing apteridine moiety linked by a methylene bridge to ...
-
CLINICAL PHARMACOLOGYFolic acid acts on megaloblastic bone marrow to produce a normoblastic marrow. In man, an exogenous source of folate is required for nucleoprotein synthesis and the maintenance of normal ...
-
INDICATIONS & USAGEFolic acid, USP is effective in the treatment of megaloblastic anemias due to a deficiency of folic acid (as may be seen in tropical or nontropical sprue) and in anemias of nutritional origin ...
-
CONTRAINDICATIONSFolic acid, USP is contraindicated in patients who have shown previous intolerance to the drug.
-
WARNINGAdministration of folic acid alone is improper therapy for pernicious anemia and other megaloblastic anemias in which vitamin B12 is deficient.
-
PRECAUTIONSGeneral - Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurologic manifestations remain progressive. There is a ...
-
ADVERSE REACTIONSAllergic sensitization has been reported following both oral and parenteral administration of folic acid. Folic acid is relatively nontoxic in man. Rare instances of allergic responses to ...
-
OVERDOSAGEExcept during pregnancy and lactation, folic acid should not be given in therapeutic doses greater than 0.4 mg daily until pernicious anemia has been ruled out. Patients with pernicious anemia ...
-
DOSAGE & ADMINISTRATIONOral administration is preferred. Although most patients with malabsorption cannot absorb food folates, they are able to absorb folic acid, USP given orally. Parenteral administration is not ...
-
HOW SUPPLIEDFolic Acid Tablets, USP 1 mg are supplied as yellow, round, biconvex uncoated tablets, debossed with "C3" on one side and breakline on other side. NDC: 72162-2413-0: 1000 Tablets in a BOTTLE - NDC ...
-
PRINCIPAL DISPLAY PANELFolic Acid 1 mg Tablet #1000
-
INGREDIENTS AND APPEARANCEProduct Information