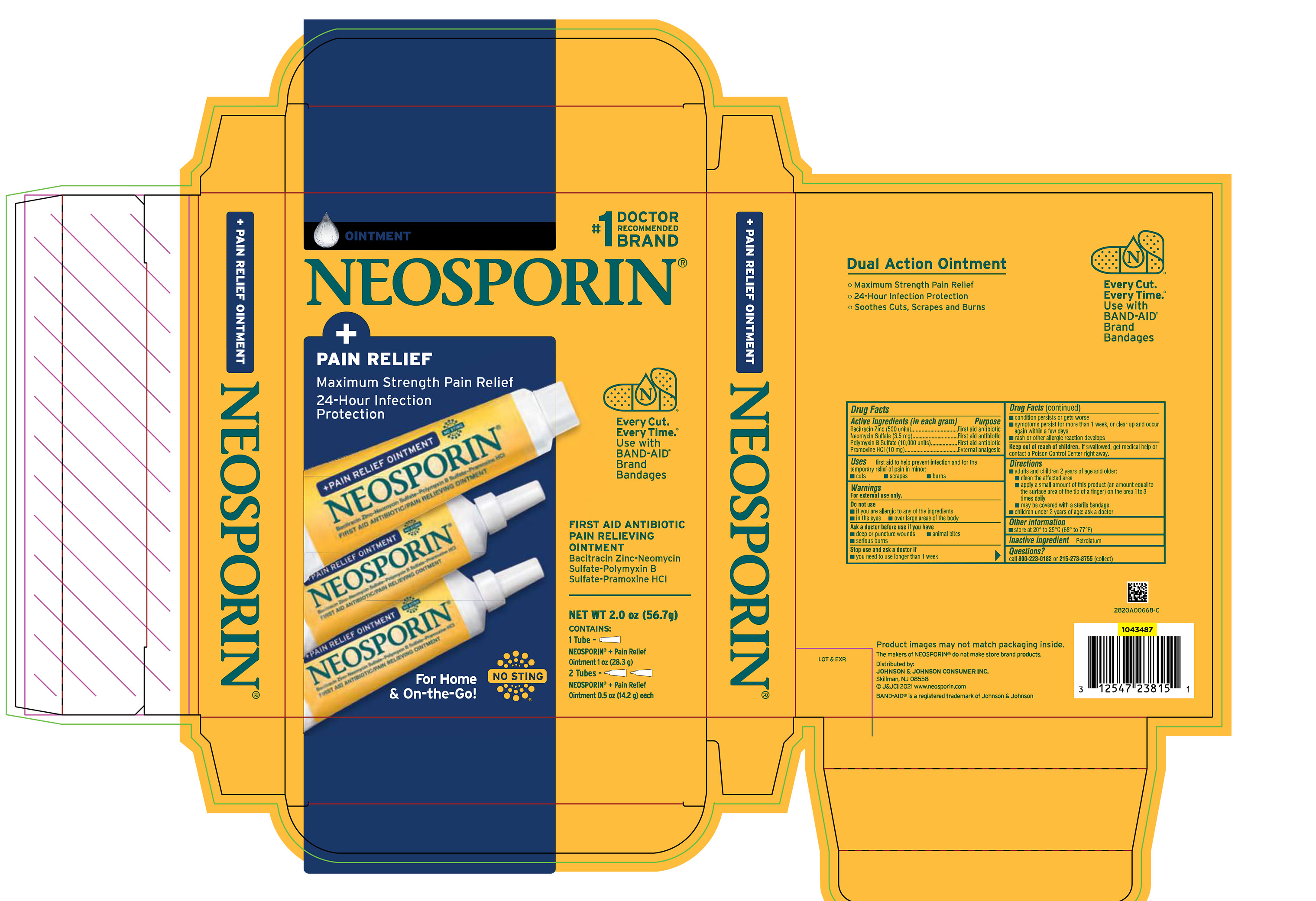
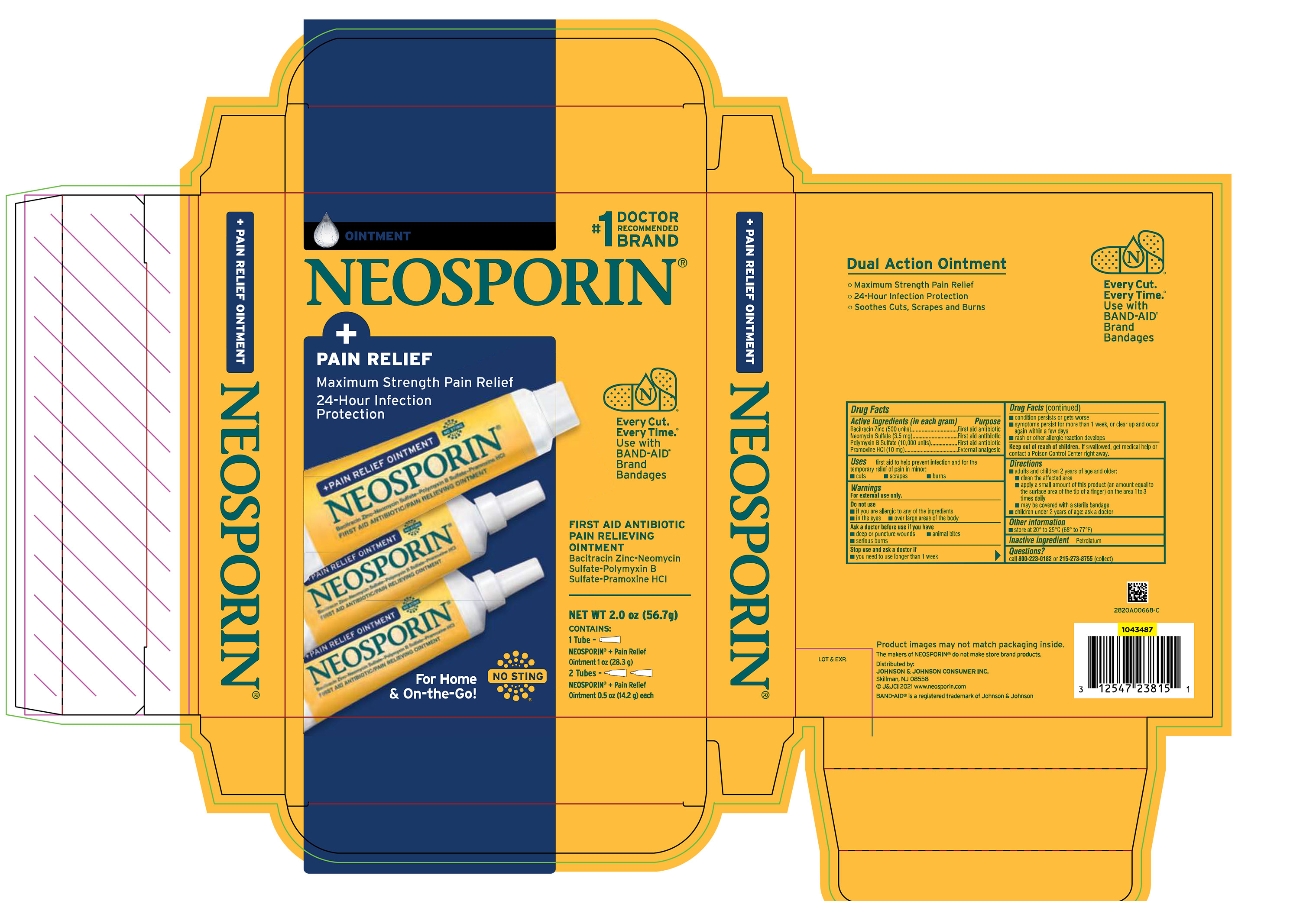
Label: NEOSPORIN PLUS PAIN RELIEF CLUBTRAY- bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride kit
- NDC Code(s): 69968-0057-1, 69968-0057-2, 69968-0801-9
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Neosporin Plus Pain Relief
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Package Label
#1 DOCTOR
RECOMMENDED
BRAND
OINTMENT
NEOSPORIN®
+
PAIN RELIEF
Maximum Strength Pain Relief
24-Hour Infection
Protection
Every Cut.
Every Time.®
Use with
BAND-AID®
Brand
Bandages
FIRST AID ANTIBIOTIC
PAIN RELIEVING
OINTMENT
Bacitracin Zinc-Neomycin
Sulfate-Polymyxin B
Sulfate-Pramoxine HCl
For Home
& On-the-Go!
NO STING
NEW WT 2.0 oz (56.7g)
CONTAINS:
1 Tube -
NEOSPORIN® + Pain Relief
Ointment 1 oz (28.3 g)
2 Tubes –
NEOSPORIN® + Pain Relief
Ointment 0.5 oz (14.2 g) each
-
INGREDIENTS AND APPEARANCE
NEOSPORIN PLUS PAIN RELIEF CLUBTRAY
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0801 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0801-9 1 in 1 PACKAGE 06/01/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 28.3 g Part 2 1 TUBE 14.2 g Part 1 of 2 NEOSPORIN PLUS PAIN RELIEF
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride ointmentProduct Information Item Code (Source) NDC:69968-0057 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0057-1 1 in 1 CARTON 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 12/01/2009 Part 2 of 2 NEOSPORIN PLUS PAIN RELIEF
bacitracin zinc, neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride ointmentProduct Information Item Code (Source) NDC:69968-0057 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 500 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0057-2 1 in 1 CARTON 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 12/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 06/01/2022 Labeler - Kenvue Brands LLC (118772437)