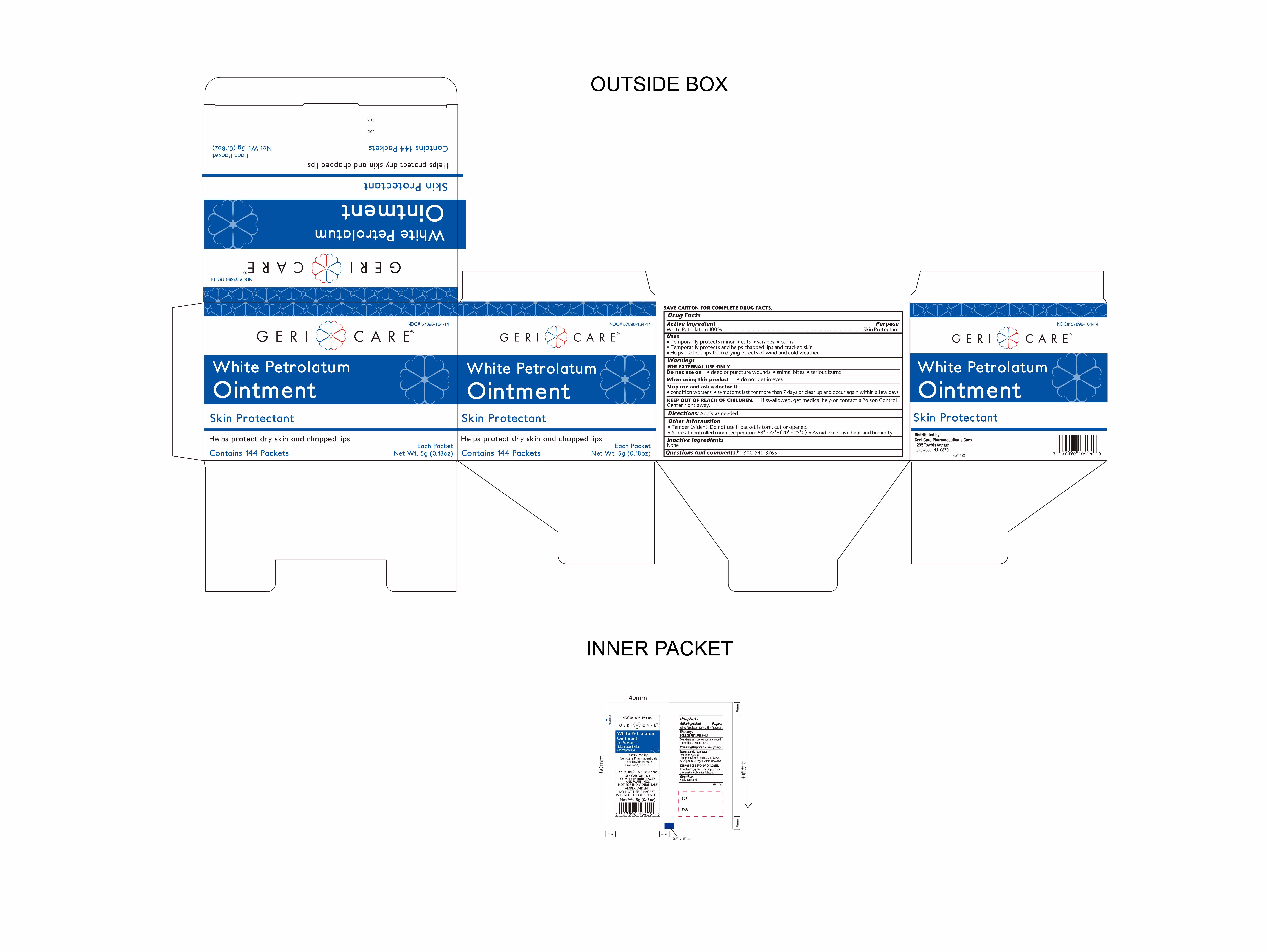
Label: WHITE PETROLATUM- petrolatum ointment
- NDC Code(s): 57896-164-14
- Packager: Geri-Care Pharmaceuticals, Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Stop use and ask a doctor
- Keep out of reach of children
- When using this product
- Inactive Ingredients:
- Do Not Use
- Directions
- SPL UNCLASSIFIED SECTION
- Warnings
- Questions
- STORAGE AND HANDLING
- Package Label
-
INGREDIENTS AND APPEARANCE
WHITE PETROLATUM
petrolatum ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57896-164 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 100 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57896-164-14 144 in 1 CARTON 01/06/2023 1 5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 01/06/2023 Labeler - Geri-Care Pharmaceuticals, Corp (611196254) Registrant - Trifecta Pharmaceuticals USA (079424163)