Label: BENZONATATE capsule
- NDC Code(s): 67296-1475-7
- Packager: Redpharm drug, inc.
- This is a repackaged label.
- Source NDC Code(s): 51224-010
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
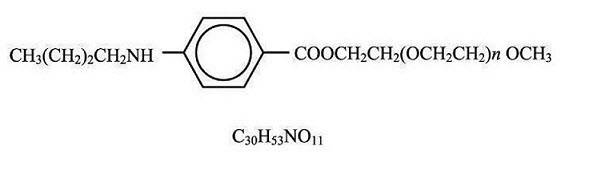
DESCRIPTIONBenzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7. Each Benzonatate ...
-
CLINICAL PHARMACOLOGYBenzonatate Capsule acts peripherally by anesthetizing the stretch receptors located in the respiratory passages, lungs, and pleura by dampening their activity and thereby reducing the cough ...
-
INDICATIONS AND USAGEBenzonatate Capsule is indicated for the symptomatic relief of cough.
-
CONTRAINDICATIONSHypersensitivity to benzonatate or related compounds.
-
WARNINGSHypersensitivity - Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from ...
-
PRECAUTIONSBenzonatate is chemically related to anesthetic agents of the para-amino-benzoic acid class (e.g. procaine; tetracaine) and has been associated with adverse CNS effects possibly related to a ...
-
ADVERSE REACTIONSPotential Adverse Reactions to Benzonatate Capsules may include: Hypersensitivity reactions including bronchospasm, laryngospasm, cardiovascular collapse possibly related to local anesthesia ...
-
OVERDOSAGEIntentional and unintentional overdose may result in death, particularly in children. The drug is chemically related to tetracaine and other topical anesthetics and shares various aspects of ...
-
DOSAGE AND ADMINISTRATIONAdults and Children over 10 years of age: Usual dose is one 100 mg or 200 mg capsule three times a day as needed for cough. If necessary to control cough, up to 600 mg daily in three divided ...
-
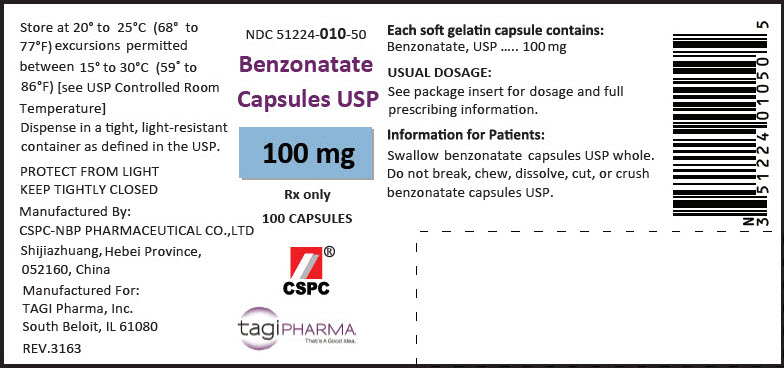
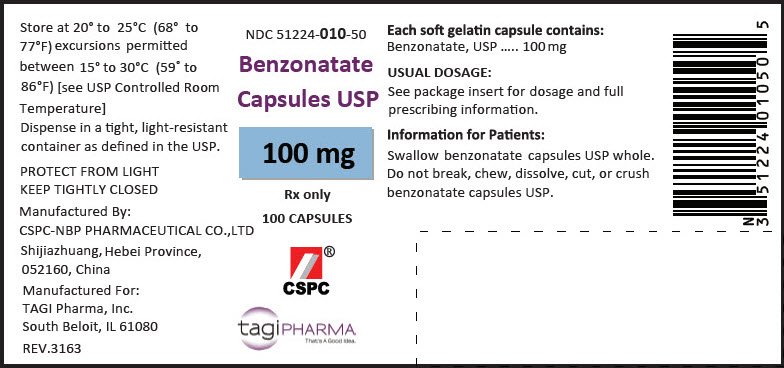
HOW SUPPLIEDBenzonatate Capsules USP , 100 mg are available as yellow, oval soft gelatin capsules with '1' imprinted in white ink. Bottles of 100: NDC 51224-010-50 - Bottles of ...
-
SPL UNCLASSIFIED SECTIONRev. 2616 - Manufactured by: CSPC-NBP Pharmaceutical Co., Ltd. Shijiazhuang, Hebei, China, 052160 - Manufactured for: TAGI Pharma, Inc ...
-
PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle LabelNDC 51224-010-50 - Benzonatate - Capsules USP - 100 mg - Rx only - 100 CAPSULES - CSPC - ® tagiPHARMA - That's A Good Idea.
-
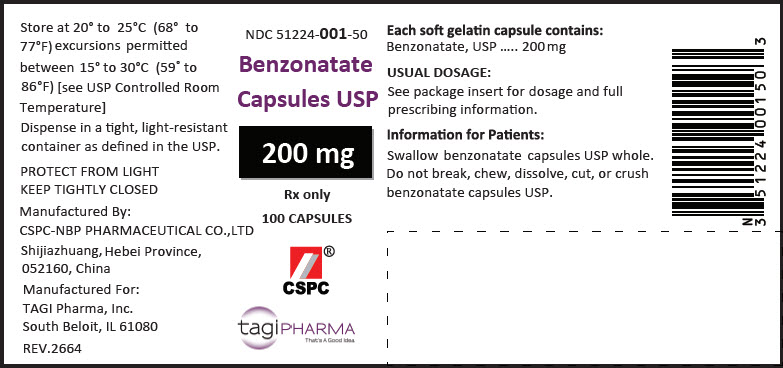
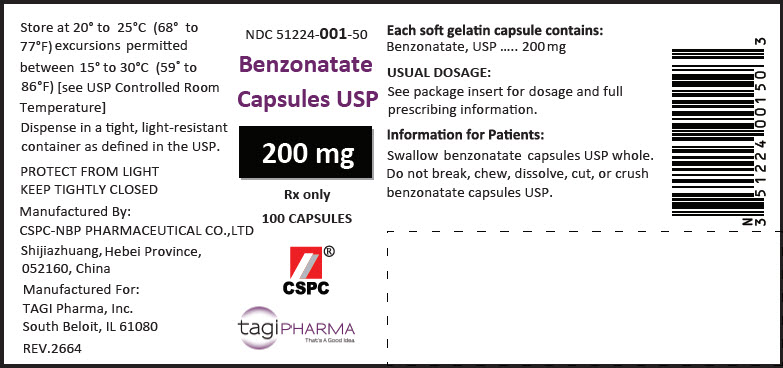
PRINCIPAL DISPLAY PANEL - 200 mg Capsule Bottle LabelNDC 51224-001-50 - Benzonatate - Capsules USP - 200 mg - Rx only - 100 CAPSULES - CSPC - ® tagiPHARMA - That's A Good Idea.
-
INGREDIENTS AND APPEARANCEProduct Information