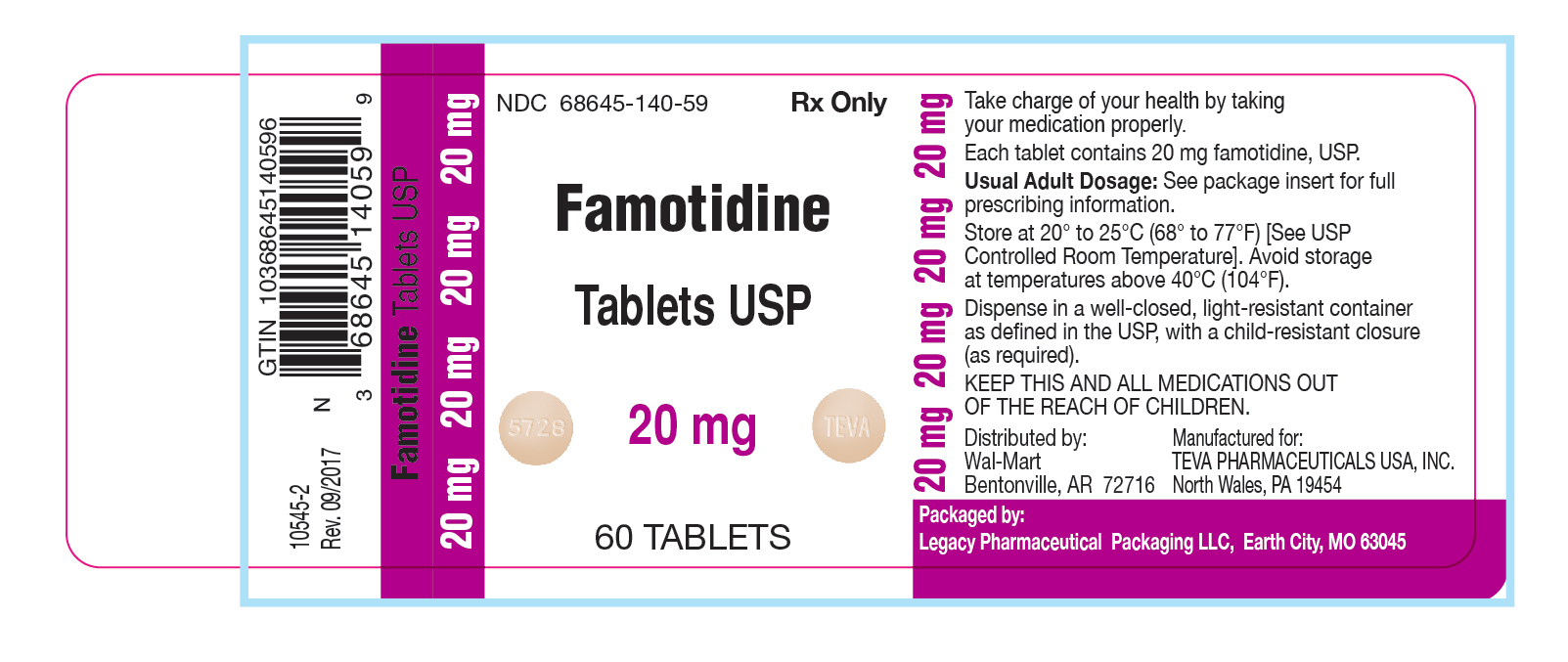
Label: FAMOTIDINE tablet, film coated
- NDC Code(s): 68645-140-59
- Packager: Legacy Pharmaceutical Packaging, LLC
- This is a repackaged label.
- Source NDC Code(s): 0172-5728
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 11, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use FAMOTIDINE TABLETS safely and effectively. See full prescribing information for FAMOTIDINE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEFamotidine tablets are indicated in adult and pediatric patients 40 kg and greater for the treatment of: active duodenal ulcer (DU). active gastric ulcer (GU). active gastric ulcer ...
-
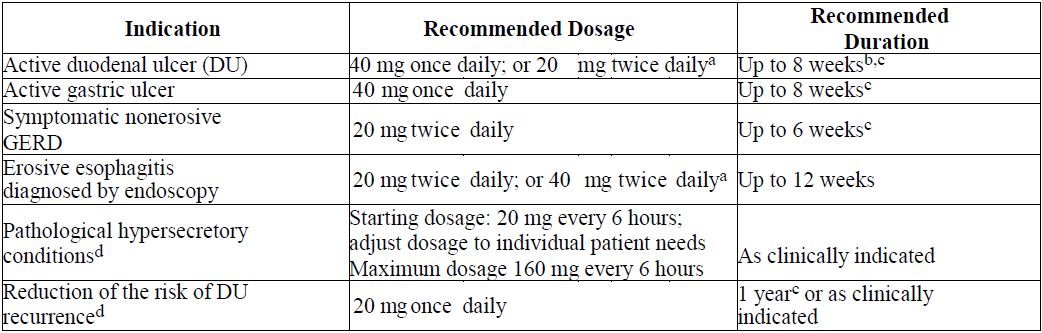
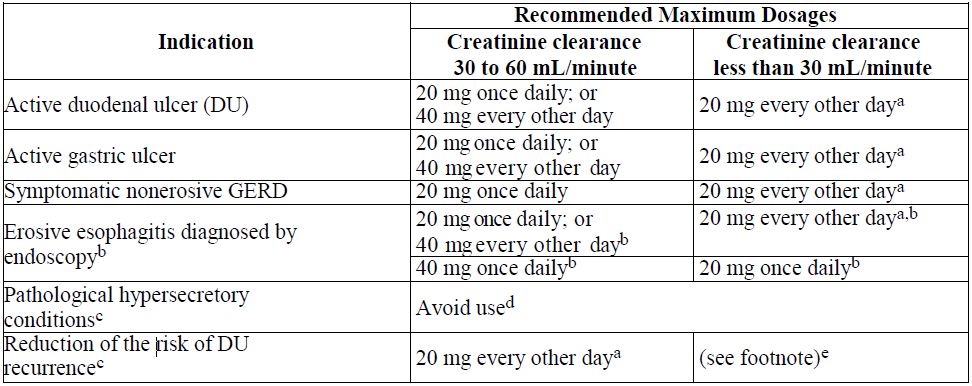
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Table 1 shows the recommended dosage of famotidine 20 mg and 40 mg tablets in adult and pediatric patients weighing 40 kg and greater with normal renal function. The use ...
-
3 DOSAGE FORMS AND STRENGTHS20 mg tablets: beige, round, unscored, film-coated tablets, debossed with "5728" on one side and "TEVA" on the other side.
-
4 CONTRAINDICATIONSFamotidine tablets are contraindicated in patients with a history of serious hypersensitivity reactions (e.g., anaphylaxis) to famotidine or other histamine-2 (H2) receptor antagonists.
-
5 WARNINGS AND PRECAUTIONS5.1 Central Nervous System Adverse Reactions - Central nervous system (CNS) adverse reactions, including confusion, delirium, hallucinations, disorientation, agitation, seizures, and lethargy ...
-
6 ADVERSE REACTIONS6.1 - Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Drugs Dependent on Gastric pH for Absorption - Famotidine can reduce the absorption of other drugs, due to its effect on reducing intragastric acidity, leading to loss of efficacy of the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data with H2-receptor antagonists, including famotidine, in pregnant women are insufficient to establish a drug-associated risk of major birth defects ...
-
10 OVERDOSAGEThe types of adverse reactions in overdosage of famotidine tablets are similar to the adverse reactions encountered with use of recommended dosages - [see Adverse Reactions (6.1)] ...
-
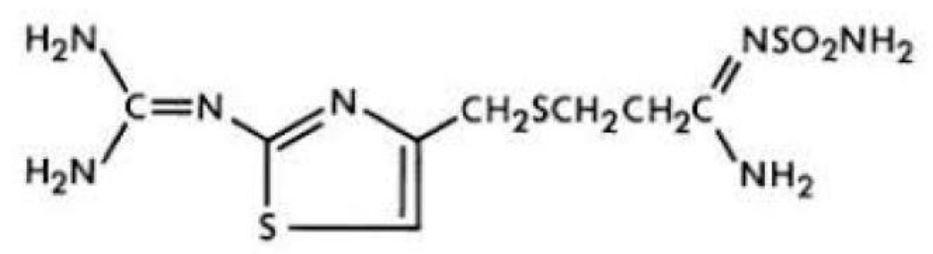
11 DESCRIPTIONThe active ingredient in Famotidine Tablets USP is a histamine-2 (H2) receptor antagonist. Famotidine, USP is [1-Amino-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio] propylidene ...
-
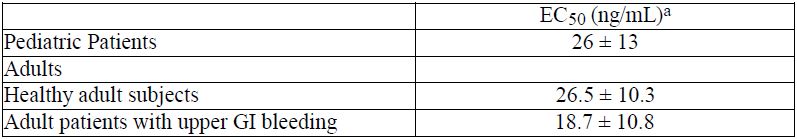
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Famotidine is a competitive inhibitor of histamine-2 (H2) receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic potential of famotidine was assessed in a 106-week oral carcinogenicity study in rats and a 92-week oral carcinogenicity ...
-
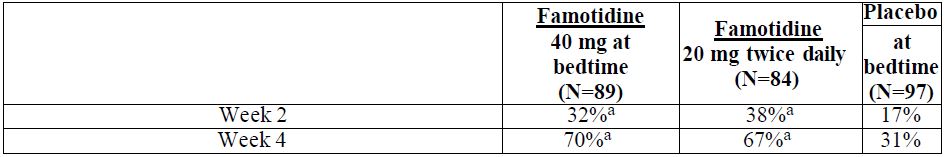
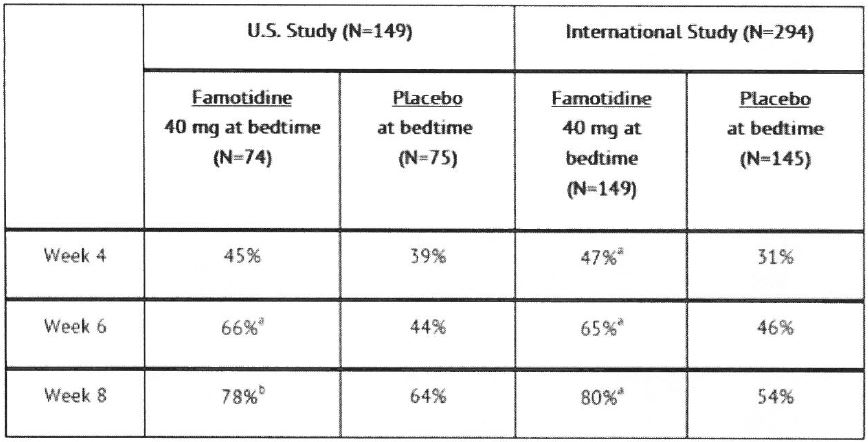
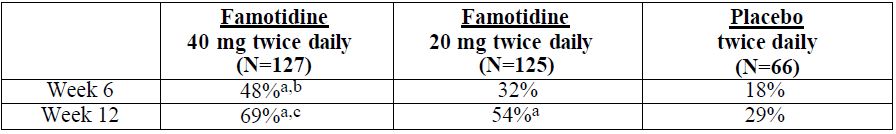
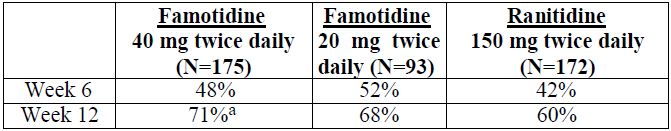
14 CLINICAL STUDIES14.1 Active Duodenal Ulcer - In a U.S. multicenter, double-blind trial in adult outpatients with endoscopically confirmed duodenal ulcer (DU), orally administered famotidine was compared to ...
-
16 HOW SUPPLIEDFamotidine Tablets USP, 20 mg are available as beige, round, unscored, film-coated tablets, debossed with "5728" on one side and “TEVA” on the other side containing 20 mg famotidine, packaged in ...
-
17 PATIENT COUNSELING INFORMATIONCentral Nervous System (CNS) Adverse Reactions - Advise elderly patients and those with moderate and severe renal impairment of the risk of CNS adverse reactions, including confusion, delirium ...
-
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information