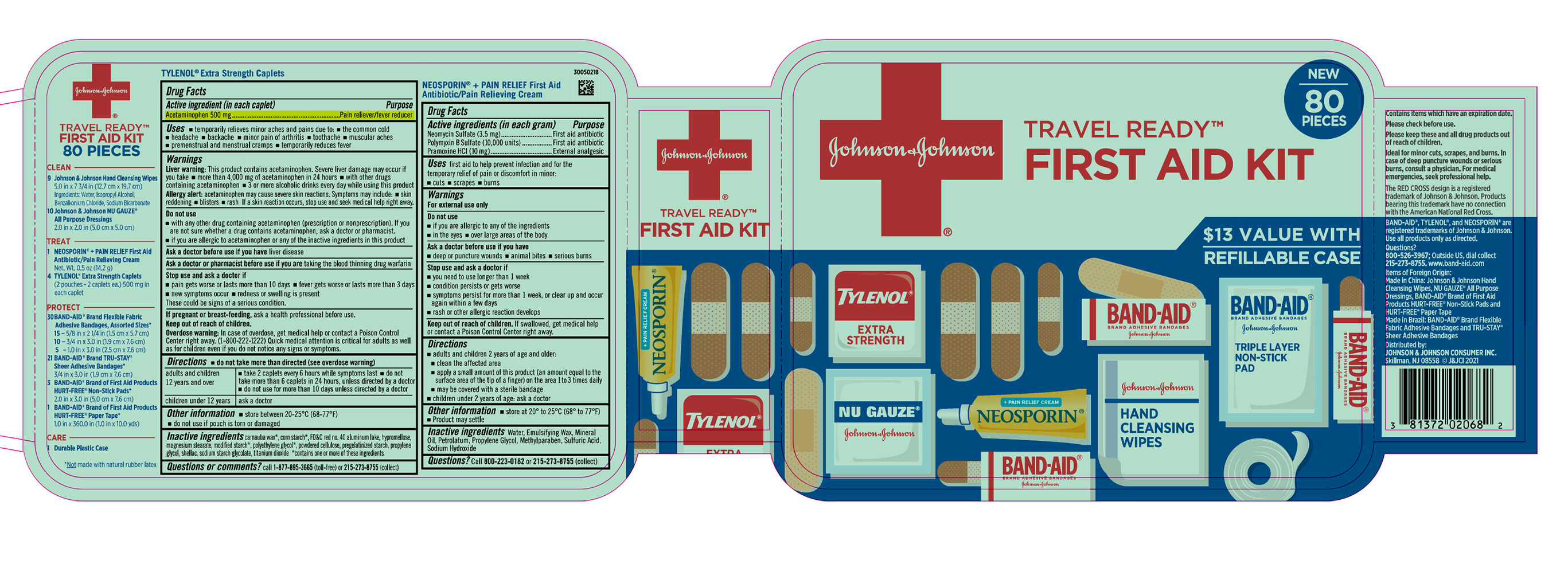
Label: JOHNSON AND JOHNSON FIRST AID TRAVEL READY 80 CT- acetaminophen, neomycin sulfate, polymyxin b sulfate, pramoxine hydrochloride kit
- NDC Code(s): 50580-449-08, 69968-0055-2, 69968-0799-9
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- TYLENOL ® Extra Strength Caplets
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- do not take more than directed (see overdose warning)
adults and children 12 years and over - take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- do not use for more than 10 days unless directed by a doctor
children under 12 years ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- NEOSPORIN ® + PAIN RELIEF First Aid Antibiotic/Pain Relieving Cream
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Package Label
-
INGREDIENTS AND APPEARANCE
JOHNSON AND JOHNSON FIRST AID TRAVEL READY 80 CT
acetaminophen, neomycin sulfate, polymyxin b sulfate, pramoxine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0799 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0799-9 1 in 1 PACKAGE 01/31/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 2 POUCH 4 Part 2 1 TUBE 14.2 g Part 3 9 PACKET 9 Part 1 of 3 TYLENOL EXTRA STRENGTH
acetaminophen tablet, film coatedProduct Information Item Code (Source) NDC:50580-449 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POWDERED CELLULOSE (UNII: SMD1X3XO9M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALUMINUM OXIDE (UNII: LMI26O6933) CARNAUBA WAX (UNII: R12CBM0EIZ) STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape OVAL Size 19mm Flavor Imprint Code TYLENOL;500 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50580-449-08 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 08/19/1984 Part 2 of 3 NEOSPORIN PLUS PAIN RELIEF FIRST AID ANTIBIOTIC/PAIN RELIEVING
neomycin sulfate, polymyxin b sulfate, and pramoxine hydrochloride creamProduct Information Item Code (Source) NDC:69968-0055 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) SULFURIC ACID (UNII: O40UQP6WCF) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0055-2 1 in 1 CARTON 1 14.2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/01/2009 Part 3 of 3 JOHNSON AND JOHNSON HAND CLEANSING WIPE
other preparations (i.e., those preparations that do not fit another category) clothProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR ISOPROPYL ALCOHOL (UNII: ND2M416302) INGR BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 05/26/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/31/2022 Labeler - Kenvue Brands LLC (118772437)