Label: AMOXICILLIN tablet, film coated
- NDC Code(s): 70518-3886-0, 70518-3886-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 57237-028
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 17, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AMOXICILLIN TABLETS safely and effectively. See full prescribing information for AMOXICILLIN TABLETS. AMOXICILLIN tablets, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAdults and Pediatric Patients - Upper Respiratory Tract Infections of the Ear, Nose, and Throat:Amoxicillin tablets are indicated in the treatment of infections due to susceptible (ONLY ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Administration Instructions - To minimize the potential for gastrointestinal intolerance, amoxicillin tablets should be taken at the start of a meal. 2.2 Dosage for Adults and ...
-
3 DOSAGE FORMS AND STRENGTHS500 mg Tabletsare pink colored, capsule shaped, film coated tablets debossed with “A” on one side and “66” on the other side.
-
4 CONTRAINDICATIONSAmoxicillin tablets are contraindicated in patients who have experienced a serious hypersensitivity reaction (e.g., anaphylaxis or Stevens-Johnson syndrome) to amoxicillin tablets or to other ...
-
5 WARNINGS AND PRECAUTIONS5.1 Anaphylactic Reactions - Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients on penicillin therapy including amoxicillin. Although ...
-
6 ADVERSE REACTIONSThe following are discussed in more detail in other sections of the labeling: Anaphylactic reactions - [see - Warnings and Precautions (5.1)] Severe Cutaneous Adverse ...
-
7 DRUG INTERACTIONS7.1 Probenecid - Probenecid decreases the renal tubular secretion of amoxicillin. Concurrent use of amoxicillin and probenecid may result in increased and prolonged blood levels of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects:Pregnancy Category B. Reproduction studies have been performed in mice and rats at doses up to 2000 mg/kg (3 and 6 times the 3 g human dose, based on body ...
-
10 OVERDOSAGEIn case of overdosage, discontinue amoxicillin, treat symptomatically, and institute supportive measures as required. A prospective study of 51 pediatric patients at a poison-control center ...
-
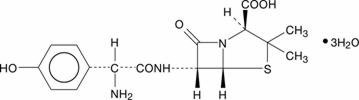
11 DESCRIPTIONAmoxicillin tablets, USP are a semisynthetic antibacterial (amoxicillin), an analog of ampicillin, with a broad spectrum of bactericidal activity against many Gram-positive and Gram-negative ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Amoxicillin is an antibacterial drug - [see - Microbiology (12.4)]. 12.3 Pharmacokinetics - Absorption:Amoxicillin is stable in the presence of gastric ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of ...
-
14 CLINICAL STUDIES14.1 - H. pyloriEradication to Reduce the Risk of Duodenal Ulcer Recurrence - Randomized, double-blind clinical studies performed in the United States in patients with - H. pyloriand duodenal ...
-
15 REFERENCESSwanson-Biearman B, Dean BS, Lopez G, Krenzelok EP. The effects of penicillin and cephalosporin ingestions in children less than six years of age. Vet Hum Toxicol. 1988; 30: 66-67.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAmoxicillin Tablets, USP contains 500 mg amoxicillin as the trihydrate. 500 mg Tablet - Pink colored, capsule shaped, film coated tablets debossed with “A” on one side and “66” on the other ...
-
17 PATIENT COUNSELING INFORMATIONAdministration Instructions - Advise patients that amoxicillin tablets may be taken every 8 hours or every 12 hours, depending on the dose prescribed. Allergic Reactions - Counsel patients that ...
-
PRINCIPAL DISPLAY PANELDRUG: Amoxicillin - GENERIC: Amoxicillin - DOSAGE: TABLET, FILM COATED - ADMINSTRATION: ORAL - NDC: 70518-3886-0 - NDC: 70518-3886-1 - COLOR: pink - SHAPE: OVAL - SCORE: No score - SIZE: 18 mm - IMPRINT ...
-
INGREDIENTS AND APPEARANCEProduct Information