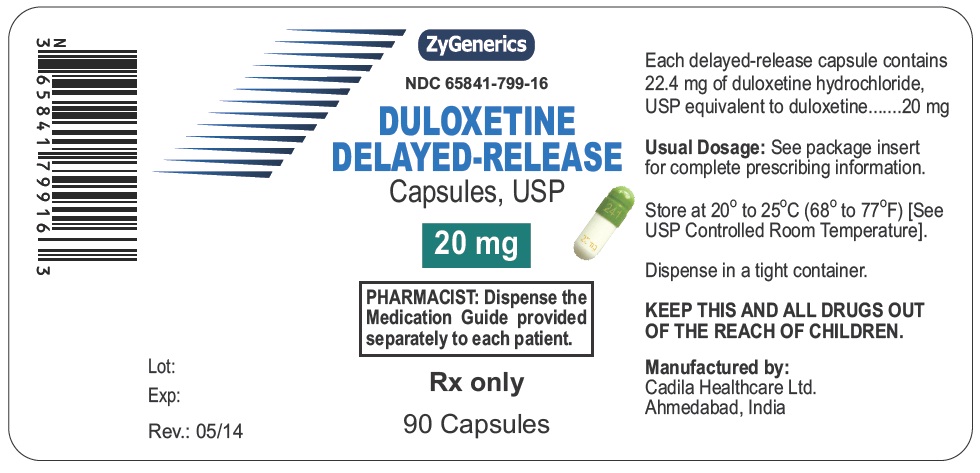
Label: DULOXETINE capsule, delayed release
-
NDC Code(s):
65841-799-06,
65841-799-14,
65841-799-16,
65841-800-06, view more65841-800-10, 65841-800-16, 65841-801-06, 65841-801-10, 65841-801-16
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DULOXETINE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-799 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 20 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMMONIA (UNII: 5138Q19F1X) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE PHTHALATE (31% PHTHALATE, 40 CST) (UNII: G4U024CQK6) HYPROMELLOSES (UNII: 3NXW29V3WO) ISOPROPYL ALCOHOL (UNII: ND2M416302) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color GREEN (GREEN) , WHITE (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 14mm Flavor Imprint Code 241;20;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-799-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 2 NDC:65841-799-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 3 NDC:65841-799-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090739 05/27/2014 DULOXETINE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-800 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 30 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) HYPROMELLOSES (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (31% PHTHALATE, 40 CST) (UNII: G4U024CQK6) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color BLUE (BLUE) , GREEN (GREEN) Score no score Shape CAPSULE (CAPSULE) Size 16mm Flavor Imprint Code 242;30;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-800-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 2 NDC:65841-800-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 3 NDC:65841-800-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090739 05/27/2014 DULOXETINE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-801 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 60 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN (UNII: 2G86QN327L) HYPROMELLOSES (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (31% PHTHALATE, 40 CST) (UNII: G4U024CQK6) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FD&C RED NO. 40 (UNII: WZB9127XOA) BUTYL ALCOHOL (UNII: 8PJ61P6TS3) ALCOHOL (UNII: 3K9958V90M) ISOPROPYL ALCOHOL (UNII: ND2M416302) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color BLUE (BLUE) , WHITE (WHITE) Score no score Shape CAPSULE (CAPSULE) Size 19mm Flavor Imprint Code 243;60;mg Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-801-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 2 NDC:65841-801-16 90 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 3 NDC:65841-801-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/27/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090739 05/27/2014 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-799, 65841-800, 65841-801) , MANUFACTURE(65841-799, 65841-800, 65841-801)