Label: AURLUMYN- iloprost injection, solution
- NDC Code(s): 83226-2001-1
- Packager: Eicos Sciences Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AURLUMYN safely and effectively. See full prescribing information for AURLUMYN. AURLUMYN™ (iloprost) injection, for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Frostbite - AURLUMYN is indicated for the treatment of severe frostbite in adults to reduce the risk of digit amputations. Effectiveness was established in young, healthy adults who suffered ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Monitor vital signs prior to the start of the infusion and with every dose increase. Administer AURLUMYN as a continuous intravenous infusion over 6 hours each day for up ...
-
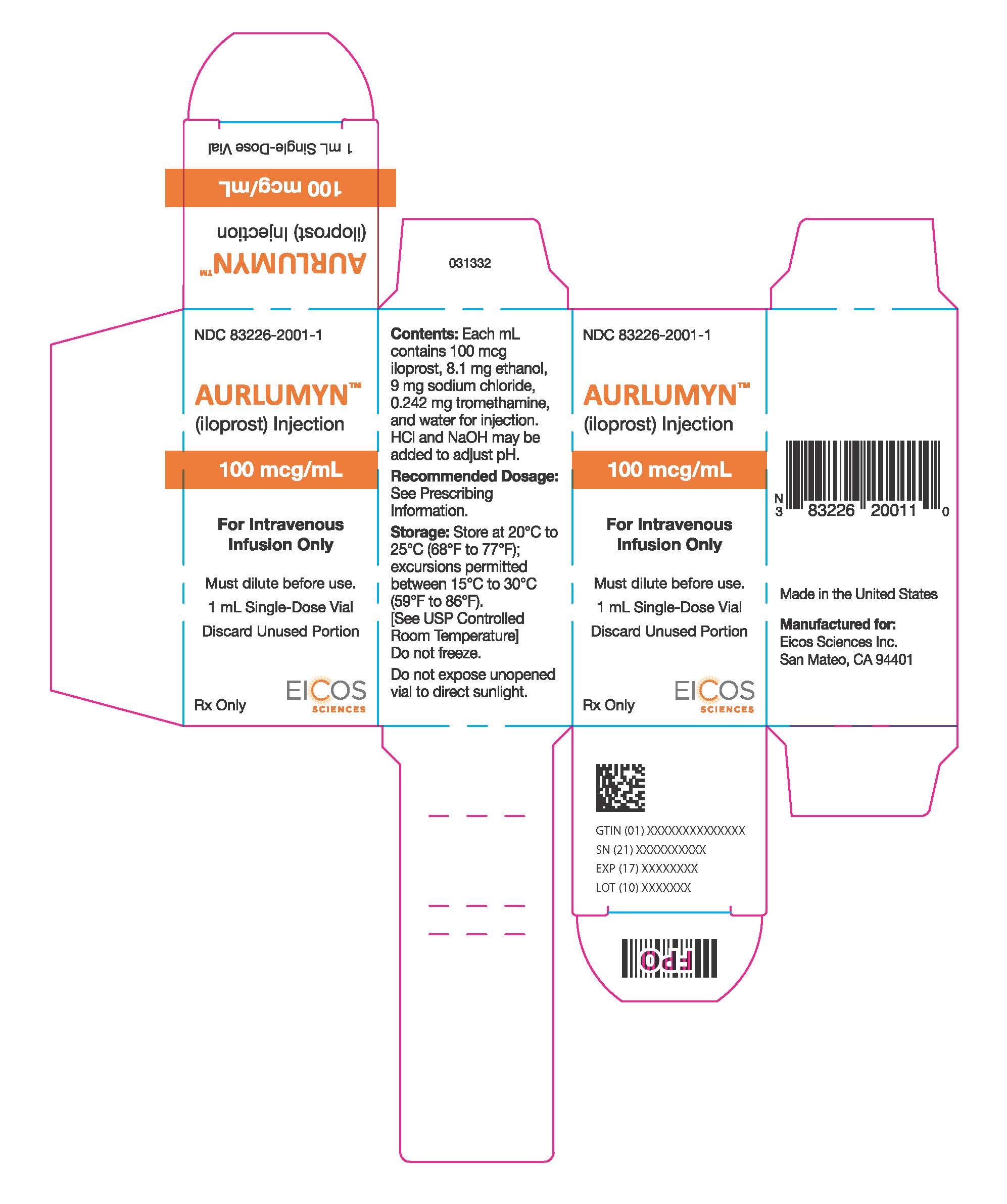
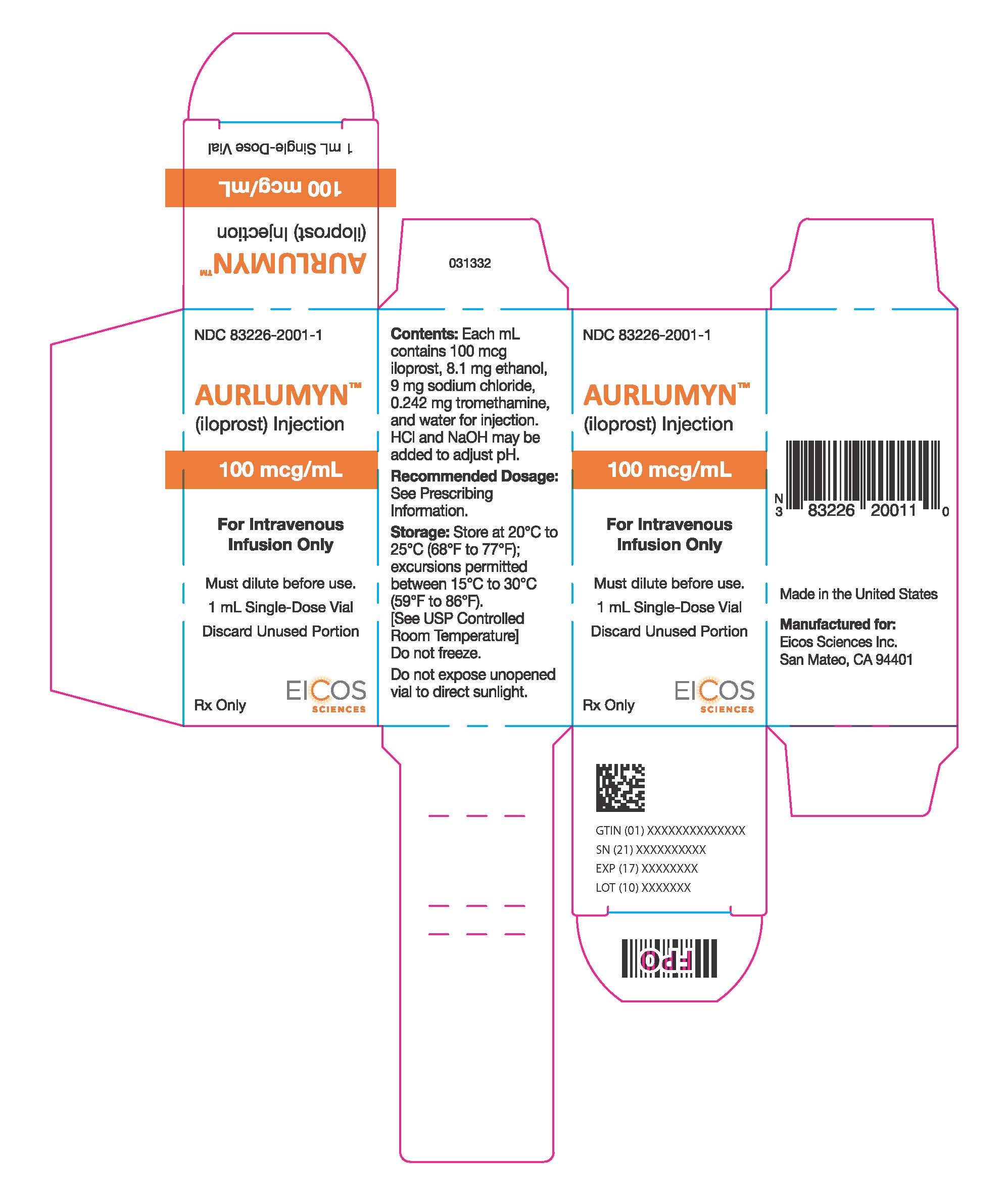
3 DOSAGE FORMS AND STRENGTHSInjection: 100 mcg per mL iloprost as a clear and colorless solution in a single-dose vial.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Hypotension - AURLUMYN is a systemic vasodilator and may cause symptomatic hypotension. Correct hypotension prior to administration of AURLUMYN. Monitor vital signs while administering ...
-
6 ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with AURMULYN during pregnancy to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or ...
-
10 OVERDOSAGECases of overdose of intravenous iloprost have not been reported. Hypotension, vomiting, and diarrhea are likely. A specific antidote is not known. Interruption of the infusion session ...
-
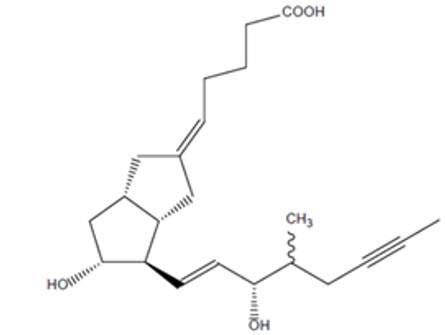
11 DESCRIPTIONAURLUMYN contains iloprost, a synthetic analog of prostacyclin PGI - 2. The chemical name for iloprost is (5 - E)-[3a - S,4 - R,5 - R,6a - S)-5-hydroxy-4-[(1 - E)-(3 - S,4 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Iloprost is a synthetic analog of prostacyclin PGI - 2. Iloprost is a vasodilator and inhibits platelet aggregation. 12.3 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Iloprost was not mutagenic in bacterial and mammalian cells in the presence or absence of extrinsic metabolic activation. Iloprost did ...
-
14 CLINICAL STUDIES14.1 Frostbite - The efficacy of intravenous (IV) iloprost for the treatment of severe frostbite to reduce the risk of digit amputations is derived from a published open-label, randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAURLUMYN (iloprost) injection is a clear, colorless sterile solution supplied as 100 mcg per mL single-dose glass vial per carton (NDC 83226-2001-1). Unopened vials of AURLUMYN are stable until ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients that they may have a fall in blood pressure with AURLUMYN, so they may become dizzy during drug administration. They should stand up slowly when they get out of a chair or ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Eicos Sciences, Inc. San Mateo, CA 94401 USA - Made in USA - ©2024 Eicos Sciences, Inc.. All rights reserved
-
PRINCIPAL DISPLAY PANEL - 1 mL Vial CartonNDC 83226-2001-1 - AURLUMYN™ (iloprost) Injection - 100 mcg/mL - For Intravenous - Infusion Only - Must dilute before use. 1 mL Single-Dose Vial - Discard Unused Portion - Rx Only - EICOS ...
-
INGREDIENTS AND APPEARANCEProduct Information