Label: DOXYCYCLINE HYCLATE powder, for solution
- NDC Code(s): 70436-032-35, 70436-032-82, 70436-201-36, 70436-201-82
- Packager: Slate Run Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Rx onlyFOR INTRAVENOUS INFUSION ONLY - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Doxycycline for Injection, USP and other antibacterial drugs, Doxycycline ...
-
DESCRIPTIONDoxycycline for Injection, USP is an antibacterial drug synthetically derived from oxytetracycline, and is available as Doxycycline Hyclate (doxycycline hydrochloride hemiethanolate hemihydrate) ...
-
CLINICAL PHARMACOLOGYTetracyclines are readily absorbed and are bound to plasma proteins in varying degree. They are concentrated by the liver in the bile, and excreted in the urine and feces at high concentrations ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of Doxycycline for Injection, USP and other antibacterial drugs, Doxycycline for Injection, USP should be used ...
-
CONTRAINDICATIONSThis drug is contraindicated in persons who have shown hypersensitivity to any of the tetracyclines.
-
WARNINGSThe use of drugs of the tetracycline class during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth ...
-
PRECAUTIONSGeneral - As with other antibacterial drugs, use of doxycycline may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, doxycycline should be discontinued ...
-
ADVERSE REACTIONSGastrointestinal - Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis and inflammatory lesions (with monilial overgrowth) in the anogenital region, and pancreatitis ...
-
DOSAGE AND ADMINISTRATIONNOTE:Rapid administration is to be avoided. Parenteral therapy is indicated only when oral therapy is not indicated. Oral therapy should be instituted as soon as possible. If intravenous therapy ...
-
HOW SUPPLIEDDoxycycline for Injection, USP, sterile powder, is supplied as follows: Unit of Sale - Units per Carton - Strength - Each - 70436-032-82 - 10 vials per carton - Doxycycline hyclate ...
-
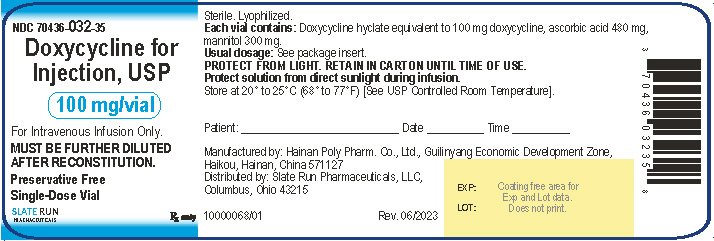
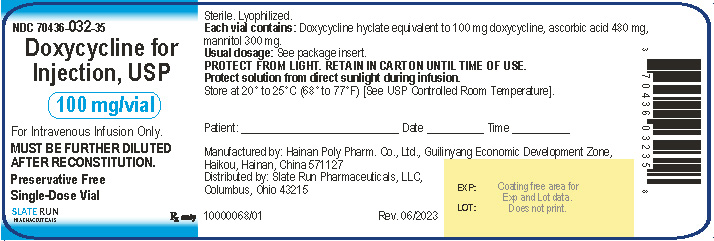
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 70436-032-35 - Doxycycline for Injection, USP, 100 mg per vial
-
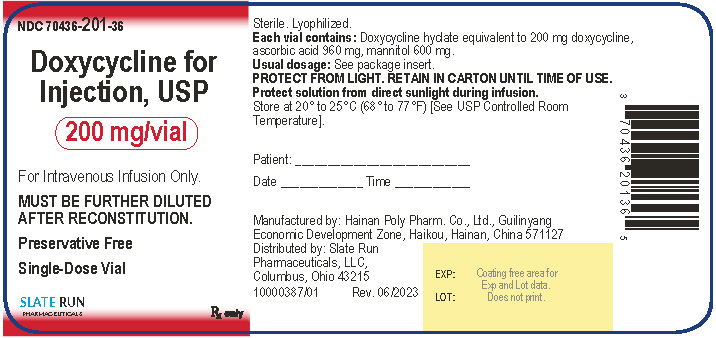
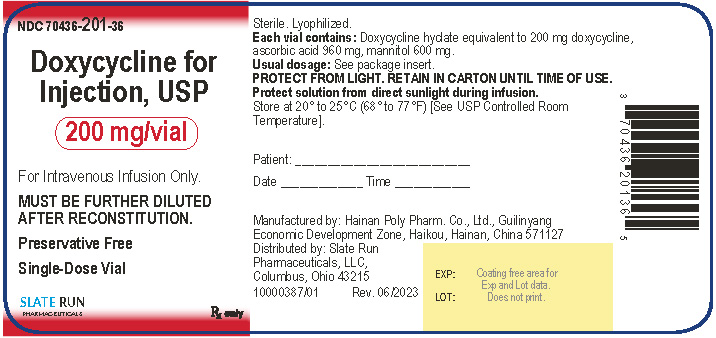
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 70436-201-36 - Doxycycline for Injection, USP, 200 mg per vial
-
INGREDIENTS AND APPEARANCEProduct Information