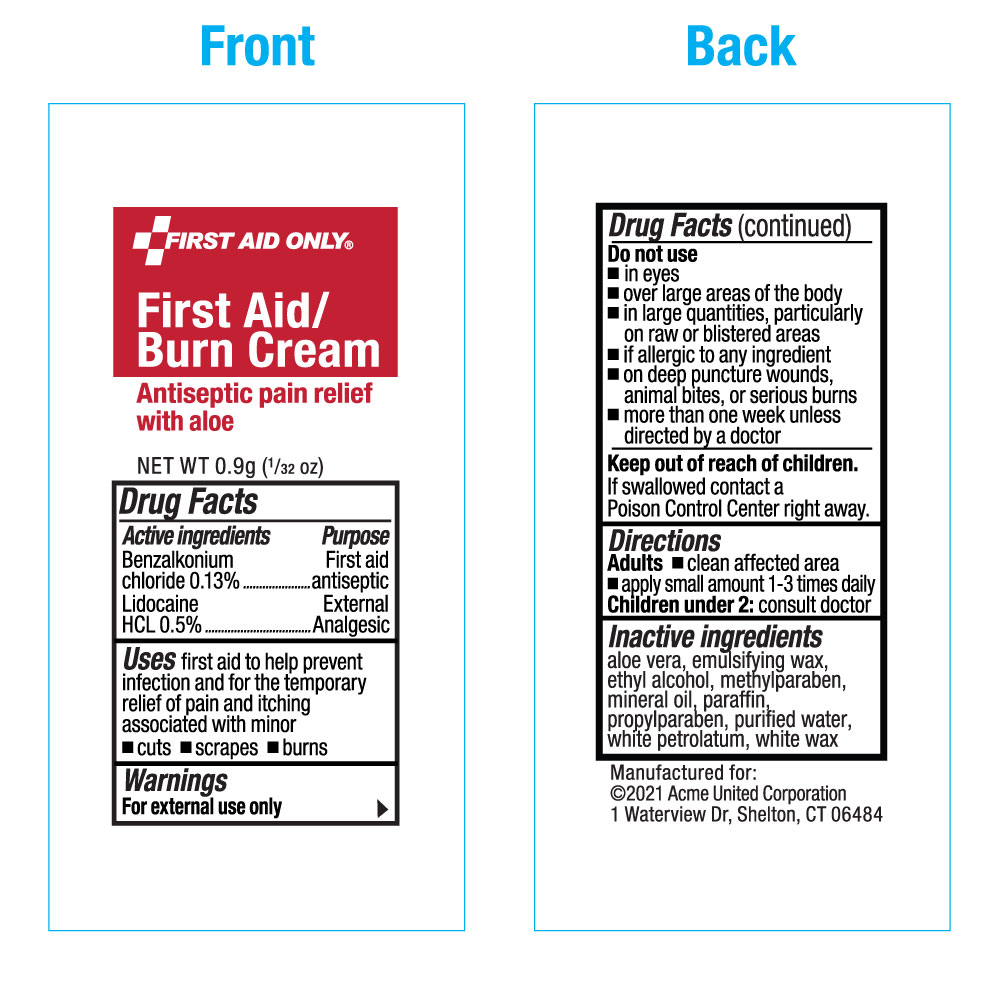
Label: FIRST AID ONLY FIRST AID-BURN- benzalkonium chloride, lidocaine hydrochloride cream
-
NDC Code(s):
0924-5702-00,
0924-5702-01,
0924-5702-02,
0924-5702-03, view more0924-5702-04, 0924-5702-05, 0924-5702-06, 0924-5702-07
- Packager: Acme United Corporation
- This is a repackaged label.
- Source NDC Code(s): 61010-5701
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FIRST AID ONLY FIRST AID-BURN
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0924-5702(NDC:61010-5701) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) PETROLATUM (UNII: 4T6H12BN9U) WHITE WAX (UNII: 7G1J5DA97F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0924-5702-00 0.9 g in 1 PACKET; Type 0: Not a Combination Product 01/30/2023 2 NDC:0924-5702-01 6 in 1 CARTON 12/31/2024 2 0.9 g in 1 PACKET; Type 0: Not a Combination Product 3 NDC:0924-5702-02 10 in 1 CARTON 12/31/2024 3 0.9 g in 1 PACKET; Type 0: Not a Combination Product 4 NDC:0924-5702-03 12 in 1 CARTON 12/31/2024 4 0.9 g in 1 PACKET; Type 0: Not a Combination Product 5 NDC:0924-5702-04 20 in 1 CARTON 12/31/2024 5 0.9 g in 1 PACKET; Type 0: Not a Combination Product 6 NDC:0924-5702-05 25 in 1 CARTON 12/31/2024 6 0.9 g in 1 PACKET; Type 0: Not a Combination Product 7 NDC:0924-5702-06 60 in 1 CARTON 12/31/2024 7 0.9 g in 1 PACKET; Type 0: Not a Combination Product 8 NDC:0924-5702-07 144 in 1 CARTON 12/31/2024 8 0.9 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/30/2023 Labeler - Acme United Corporation (001180207) Establishment Name Address ID/FEI Business Operations Acme United Corporation 045924339 relabel(0924-5702) , repack(0924-5702) Establishment Name Address ID/FEI Business Operations Acme United Corporation 080119599 relabel(0924-5702) , repack(0924-5702)