Label: ZONTIVITY- vorapaxar tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 66992-208-30, 66992-208-90 - Packager: WraSer Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated October 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZONTIVITY safely and effectively. See full prescribing information for ZONTIVITY. ZONTIVITY - ® (vorapaxar) Tablets 2.08 ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: BLEEDING RISK
- Do not use ZONTIVITY in patients with a history of stroke, transient ischemic attack (TIA), or intracranial hemorrhage (ICH); or active pathological bleeding [see CONTRAINDICATIONS (4.1, 4.2)] .
- Antiplatelet agents, including ZONTIVITY, increase the risk of bleeding, including ICH and fatal bleeding [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE1.1 Patients with History of Myocardial Infarction (MI) or with Peripheral Arterial Disease (PAD) ZONTIVITY - ® is indicated for the reduction of thrombotic cardiovascular ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - Take one tablet of ZONTIVITY 2.08 mg orally once daily, with or without food. 2.2 Coadministration with Other Antiplatelet Drugs - There is no ...
-
3 DOSAGE FORMS AND STRENGTHSZONTIVITY tablets, 2.08 mg vorapaxar, are yellow, oval-shaped, film-coated tablets with "351" on one side.
-
4 CONTRAINDICATIONS4.1 History of Stroke, Transient Ischemic Attack (TIA), or Intracranial Hemorrhage (ICH) ZONTIVITY is contraindicated in patients with a history of stroke, TIA, or ICH because of an ...
-
5 WARNINGS AND PRECAUTIONS5.1 General Risk of Bleeding - Antiplatelet agents, including ZONTIVITY, increase the risk of bleeding, including ICH and fatal bleeding - [see - Adverse ...
-
6 ADVERSE REACTIONSThe following serious adverse reaction is also discussed elsewhere in the labeling: Bleeding [see - Boxed Warning and - Warnings and Precautions (5.1) ...
-
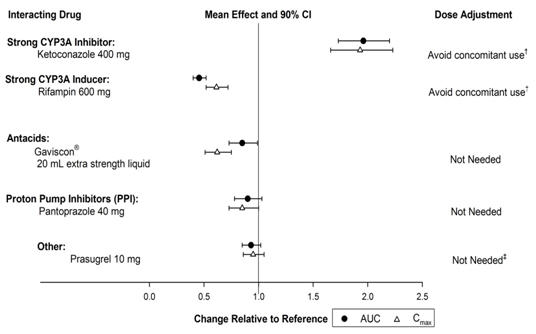
7 DRUG INTERACTIONS7.1 Effects of Other Drugs on ZONTIVITY - Vorapaxar is eliminated primarily by metabolism, with contributions from CYP3A4 and CYP2J2. Strong CYP3A Inhibitors - Avoid concomitant use of ...
-
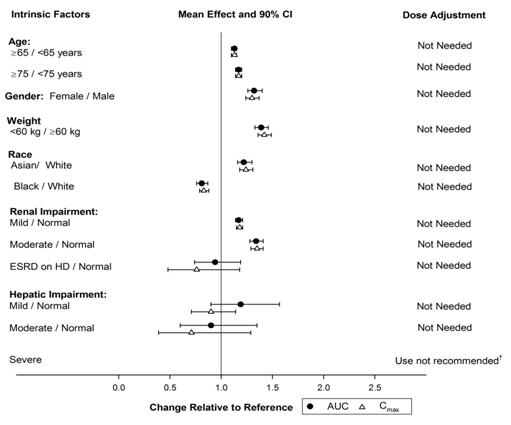
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on the potential for serious adverse reactions (such as maternal bleeding/hemorrhage) and the long half-life which makes it effectively irreversible ...
-
10 OVERDOSAGEThere is no known treatment to reverse the antiplatelet effect of ZONTIVITY, and neither dialysis nor platelet transfusion can be expected to be beneficial if bleeding occurs after overdose ...
-
11 DESCRIPTIONZONTIVITY contains vorapaxar sulfate, a tricyclic himbacine-derived selective inhibitor of platelet aggregation mediated by PAR-1. The chemical name of vorapaxar sulfate is ethyl ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Vorapaxar is a reversible antagonist of the protease-activated receptor-1 (PAR-1) expressed on platelets, but its long half-life makes it effectively irreversible ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Carcinogenicity studies were conducted in rats and mice dosed orally with vorapaxar for two years. Male and ...
-
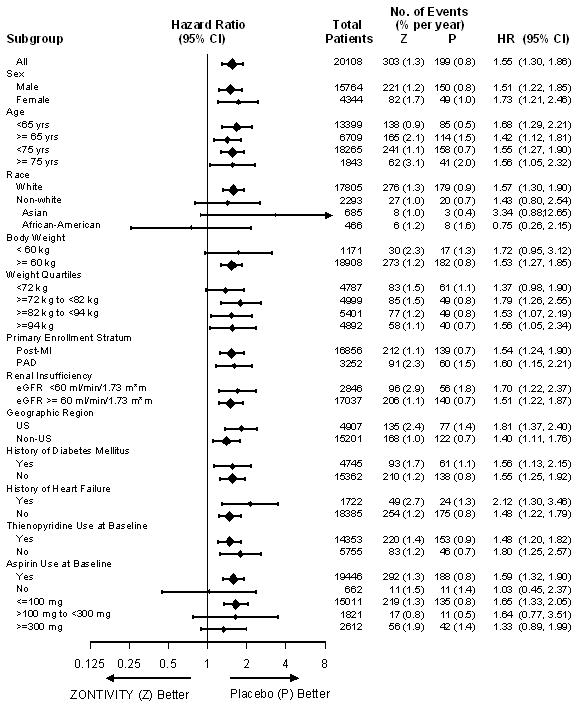
14 CLINICAL STUDIESThe clinical evidence for the effectiveness of ZONTIVITY is supported by TRA 2°P - TIMI 50. TRA 2°P was a multicenter, randomized, double-blind, placebo-controlled study conducted in patients who ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGZONTIVITY tablets, 2.08 mg vorapaxar, are yellow, oval-shaped, film-coated tablets with "351" on one side. They are supplied as follows: NDC 66992-208-30 bottles of 30 tablets - NDC 66992-208-90 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved Patient Labeling ( Medication Guide). Benefits and Risks - Summarize the benefits and potential side effects of ...
-
SPL UNCLASSIFIED SECTIONManufactured for: WraSer Pharmaceuticals - Ridgeland, MS 39157 - www.wraser.com
-
MEDICATION GUIDEMedication Guide - ZONTIVITY - ® (zon-TIV-iti) (vorapaxar) Tablets - Read this ...
-
SPL UNCLASSIFIED SECTIONManufactured for: WraSer Pharmaceuticals - Ridgeland MS 39157 - www.wraser.com - Revised: 10/2022
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label - NDC 66992-208-30 - Zontivity - ® (vorapaxar) tablets - 2.08 mg* Dispense the accompanying - Medication ...
-
INGREDIENTS AND APPEARANCEProduct Information