Label: SODIUM CHLORIDE injection, solution
- NDC Code(s): 0264-7805-10, 0264-7806-10
- Packager: B. Braun Medical Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each 100 mL of 3% Sodium Chloride Injection USP contains:
Sodium Chloride USP 3 g; Water for Injection USP qs
pH: 5.8 (4.5–7.0) Calculated Osmolarity: 1030 mOsmol/liter
pH may be adjusted with Hydrochloric Acid NFConcentration of Electrolytes (mEq/liter): Sodium 513 Chloride 513
Each 100 mL of 5% Sodium Chloride Injection USP contains:
Sodium Chloride USP 5 g; Water for Injection USP qs
pH: 5.8 (4.5–7.0) Calculated Osmolarity: 1710 mOsmol/liter
pH may be adjusted with Hydrochloric Acid NFConcentration of Electrolytes (mEq/liter): Sodium 856 Chloride 856
3% and 5% Sodium Chloride Injections USP are sterile, nonpyrogenic and contain no bacteriostatic or antimicrobial agents.
The formula of the active ingredient is:
Ingredient Molecular Formula Molecular Weight Sodium Chloride USP NaCl 58.44 Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
Addition of medication should be accomplished using complete aseptic technique.
The closure system has two ports; the one for the administration set has a tamper evident plastic protector and the other is a medication addition site. Refer to the Directions for Use of the container.
-
CLINICAL PHARMACOLOGY
3% and 5% Sodium Chloride Injections USP provide electrolytes and are a source of water for hydration. They are capable of inducing diuresis depending on the clinical condition of the patient.
Sodium, the major cation of the extracellular fluid, functions primarily in the control of water distribution, fluid balance, and osmotic pressure of body fluids. Sodium is also associated with chloride and bicarbonate in the regulation of the acid-base equilibrium of body fluid.
Chloride, the major extracellular anion, closely follows the metabolism of sodium, and changes in the acid-base balance of the body are reflected by changes in the chloride concentration.
-
INDICATIONS AND USAGE
These intravenous solutions are indicated for use in adults and pediatric patients as sources of electrolytes and water for hydration.
3% and 5% Sodium Chloride Injections USP are of particular value in severe salt depletion when rapid electrolyte restoration is of paramount importance. The low salt syndrome may occur in the presence of heart failure, renal impairment, during surgery, and postoperatively. In these conditions, chloride loss frequently exceeds sodium loss.
These hypertonic sodium chloride solutions are also indicated for the following clinical conditions.
- Hyponatremia and hypochloremia due to electrolyte and fluid loss replaced with sodium-free fluids.
- Drastic dilution of extracellular body fluid following excessive water intake sometimes resulting from multiple enemas or perfusion of irrigating fluids into open venous sinuses during transurethral prostatic resections.
- Emergency treatment of severe salt depletion due to excess sweating, vomiting, diarrhea and other conditions.
- CONTRAINDICATIONS
-
WARNINGS
Caution: These are concentrated hypertonic sodium chloride solutions. Infuse very slowly with constant observation of the patient to avoid pulmonary edema.
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration.
Solutions containing sodium ions should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there is sodium retention with edema. In patients with diminished renal function, administration of solutions containing sodium ions may result in sodium retention.
-
PRECAUTIONS
General
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
These solutions should be used with care in patients with hypervolemia, renal insufficiency, urinary tract obstruction, or impending or frank cardiac decompensation.
Extraordinary electrolyte losses such as may occur during protracted nasogastric suction, vomiting, diarrhea or gastrointestinal fistula drainage may necessitate additional electrolyte supplementation.
Additional essential electrolytes, minerals and vitamins should be supplied as needed.
Sodium-containing solutions should be administered with caution to patients receiving corticosteroids or corticotropin, or to other salt-retaining patients. Care should be exercised in administering solutions containing sodium to patients with renal or cardiovascular insufficiency, with or without congestive heart failure, particularly if they are postoperative or elderly.
Excessive infusion of hypertonic sodium chloride solutions may supply more sodium and chloride than normally found in serum and can exceed normal tolerance, resulting in hypernatremia. Infusion of excess chloride ions may cause a loss of bicarbonate, resulting in an acidifying effect.
To minimize the risk of possible incompatibilities arising from mixing this solution with other additives that may be prescribed, the final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
Do not use plastic containers in series connection.
If administration is controlled by a pumping device, care must be taken to discontinue pumping action before the container runs dry or air embolism may result. If administration is not controlled by a pumping device, refrain from applying excessive pressure (>300mmHg) causing distortion to the container such as wringing or twisting. Such handling could result in breakage of the container.
These solutions are intended for intravenous administration using sterile equipment. It is recommended that intravenous administration apparatus be replaced at least once every 24 hours.
Use only if solution is clear and container and seals are intact.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with 3% and 5% Sodium Chloride Injections USP have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with 3% and 5% Sodium Chloride Injections USP. It is also not known whether 3% and 5% Sodium Chloride Injections USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. 3% and 5% Sodium Chloride Injections USP should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when 3% and 5% Sodium Chloride Injections USP are administered to a nursing woman.
Pediatric Use
Safety and effectiveness of sodium chloride injections in pediatric patients have not been established by adequate and well controlled trials, however, the use of electrolyte solutions in the pediatric population is referenced in the medical literature. The warnings, precautions and adverse reactions identified in the label copy should be observed in the pediatric population.
Geriatric Use
An evaluation of current literature revealed no clinical experience identifying differences in response between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
Too rapid infusion of hypertonic solutions may cause local pain and venous irritation. Rate of administration should be adjusted according to tolerance. Use of the largest peripheral vein and a well-placed small bore needle is recommended. (See DOSAGE AND ADMINISTRATION.)
The physician should also be alert to the possibility of adverse reactions to drug additives. Prescribing information for drug additives to be administered in this manner should be consulted.
Symptoms may result from an excess or deficit of one or more of the ions present in the solution; therefore, frequent monitoring of electrolyte levels is essential.
Hypernatremia may be associated with edema and exacerbation of congestive heart failure due to the retention of water, resulting in an expanded extracellular fluid volume.
If infused in large amounts, chloride ions may cause a loss of bicarbonate ions, resulting in an acidifying effect.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
These solutions are for intravenous use only.
Dosage is to be directed by a physician and is dependent upon age, weight, clinical condition of the patient and laboratory determinations. Frequent laboratory determinations and clinical evaluation are essential to monitor changes in blood glucose and electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
When a hypertonic solution is to be administered peripherally, it should be slowly infused through a small bore needle, placed well within the lumen of a large vein to minimize venous irritation. Carefully avoid infiltration.
Maximum intravenous dosage should be 100 mL given over a period of one hour. Before additional amounts are given, the serum electrolyte concentrations, including chloride and bicarbonate, should be determined to evaluate the need for more sodium chloride.
Intravenous administration of these solutions should not exceed 100 mL/hour or 400 mL/24 hours.
Fluid administration should be based on calculated maintenance or replacement fluid requirements for each patient.
Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
-
HOW SUPPLIED
3% and 5% Sodium Chloride Injections USP are supplied sterile and nonpyrogenic in EXCEL® Containers packaged 24 per case.
NDC REF Size 3% Sodium Chloride Injection USP 0264-7805-10 L8051 500 mL 5% Sodium Chloride Injection USP
(Canada DIN 01928007)0264-7806-10 L8061 500 mL Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. Protect from freezing. It is recommended that the product be stored at room temperature (25°C); however, brief exposure up to 40°C does not adversely affect the product.
Storage in automated dispensing machines: Brief exposure up to 2 weeks to ultraviolet or fluorescent light does not adversely affect the product labeling legibility; prolonged exposure can cause fading of the red label. Rotate stock frequently.
- SPL UNCLASSIFIED SECTION
-
Directions for Use of EXCEL® Container
Caution: Do not use plastic containers in series connection.
To Open
Tear overwrap down at notch and remove solution container. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired. If supplemental medication is desired, follow directions below before preparing for administration.
NOTE: Before use, perform the following checks:
- Inspect each container. Read the label. Ensure solution is the one ordered and is within the expiration date.
- Invert container and carefully inspect the solution in good light for cloudiness, haze, or particulate matter. Any container which is suspect should not be used.
- Use only if solution is clear and container and seals are intact.
Preparation for Administration
- Remove plastic protector from sterile set port at bottom of container.
- Attach administration set. Refer to complete directions accompanying set.
To Add Medication
Warning: Some additives may be incompatible.
To Add Medication Before Solution Administration
- Prepare medication site.
- Using syringe with 18–22 gauge needle, puncture medication port and inner diaphragm and inject.
- Squeeze and tap ports while ports are upright and mix solution and medication thoroughly.
To Add Medication During Solution Administration
- Close clamp on the set.
- Prepare medication site.
- Using syringe with 18–22 gauge needle of appropriate length (at least 5/8 inch), puncture resealable medication port and inner diaphragm and inject.
- Remove container from IV pole and/or turn to an upright position.
- Evacuate both ports by tapping and squeezing them while container is in the upright position.
- Mix solution and medication thoroughly.
- Return container to in use position and continue administration.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 500 mL
3% Sodium Chloride
Injection USPREF L8051
NDC 0264-7805-10
500 mL
EXCEL® CONTAINER
Hypertonic
Y94-003-261 LD-259-2Each 100 mL contains: Sodium Chloride USP 3 g; Water for Injection USP qs
pH may be adjusted with HCI NF
pH: 5.8 (4.5-7.0); Calc. Osmolarity: 1030 mOsmol/liter
Electrolytes (mEq/liter): Na+ 513; CI– 513
Sterile, nonpyrogenic. Single dose container.
CAUTION: This is a concentrated sodium chloride solution. Infuse slowly with
constant observation of patient to avoid pulmonary edema.
Do not use in series connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.
WARNINGS: Some additives may be incompatible. Consult with pharmacist. When
introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive heat. Protect from
freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
Y94-003-260
LD-133-3
EXP
LOT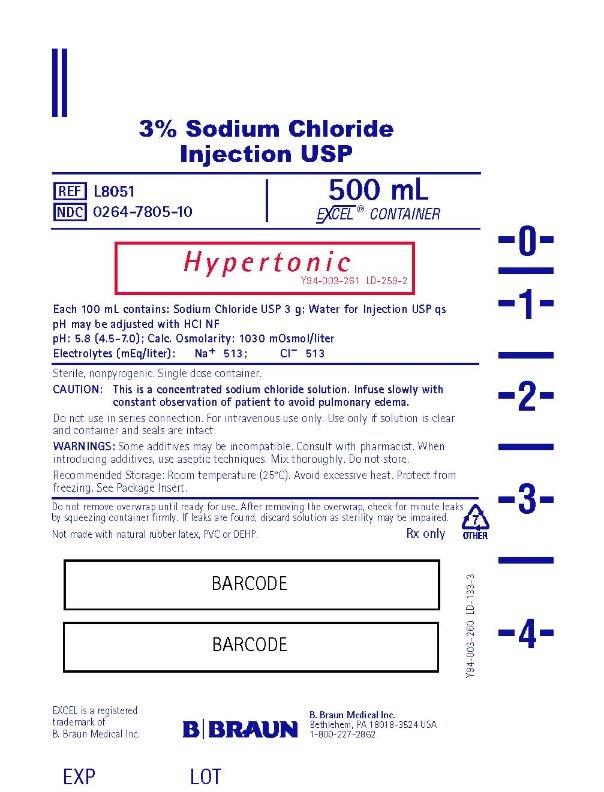
L8051
-
PRINCIPAL DISPLAY PANEL - 500 mL
5% Sodium Chloride
Injection USP
REF L8061
NDC 0264-7806-10
DIN 01928007
HK 22618
500 mL
EXCEL® CONTAINER
Hypertonic
Y94-003-269 LD-501-1
Each 100 mL contains: Sodium Chloride USP 5 g; Water for Injection USP qs
pH may be adjusted with HCI NF
pH: 5.8 (4.5-7.0) ; Calc. Osmolarity: 1710 mOsmol/liter
Electrolytes (mEq/liter): Na+ 856; CI– 856
Sterile, nonpyrogenic. Single dose container.
CAUTION: This is a concentrated sodium chloride solution. Infuse slowly with
constant observation of patient to avoid pulmonary edema.
Do not use in series connection. For intravenous use only. Use only if solution is clear
and container and seals are intact.
WARNINGS: Some additives may be incompatible. Consult with pharmacist. When
introducing additives, use aseptic techniques. Mix thoroughly. Do not store.
Recommended Storage: Room temperature (25°C). Avoid excessive heat. Protect from
freezing. See Package Insert.Do not remove overwrap until ready for use. After removing the overwrap, check for minute leaks by squeezing container firmly. If leaks are found, discard solution as sterility may be impaired.
Not made with natural rubber latex, PVC or DEHP.
Rx only

EXCEL is a registered trademark of B. Braun Medical Inc.B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
1-800-227-2862
In Canada, distributed by:
B. Braun of Canada, Ltd.
Scarborough, Ontario M1H 2W4
Y94-003-262
LD-132-3
EXP
LOT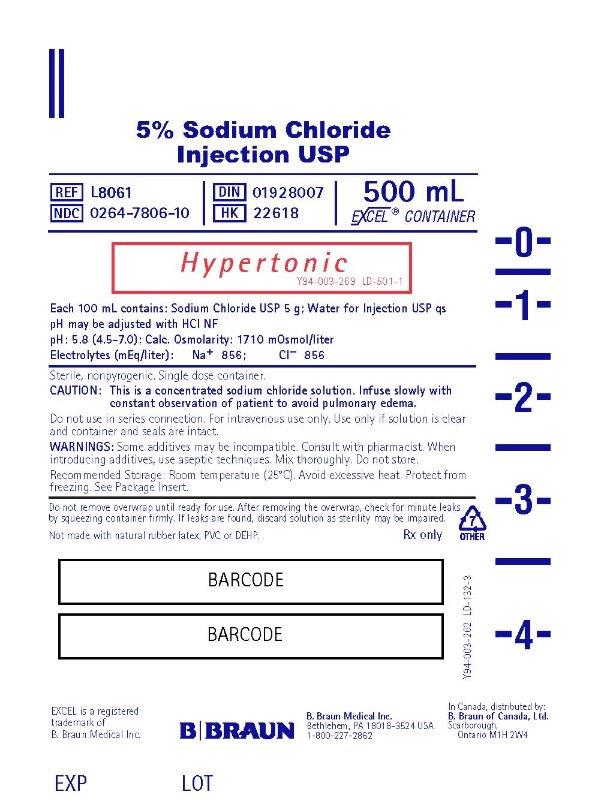
L8061
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-7805 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-7805-10 24 in 1 CASE 03/19/1988 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019635 03/19/1988 SODIUM CHLORIDE
sodium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0264-7806 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0264-7806-10 24 in 1 CASE 03/09/1988 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019635 03/09/1988 Labeler - B. Braun Medical Inc. (002397347) Establishment Name Address ID/FEI Business Operations B. Braun Medical Inc. 037425308 manufacture(0264-7805, 0264-7806)




