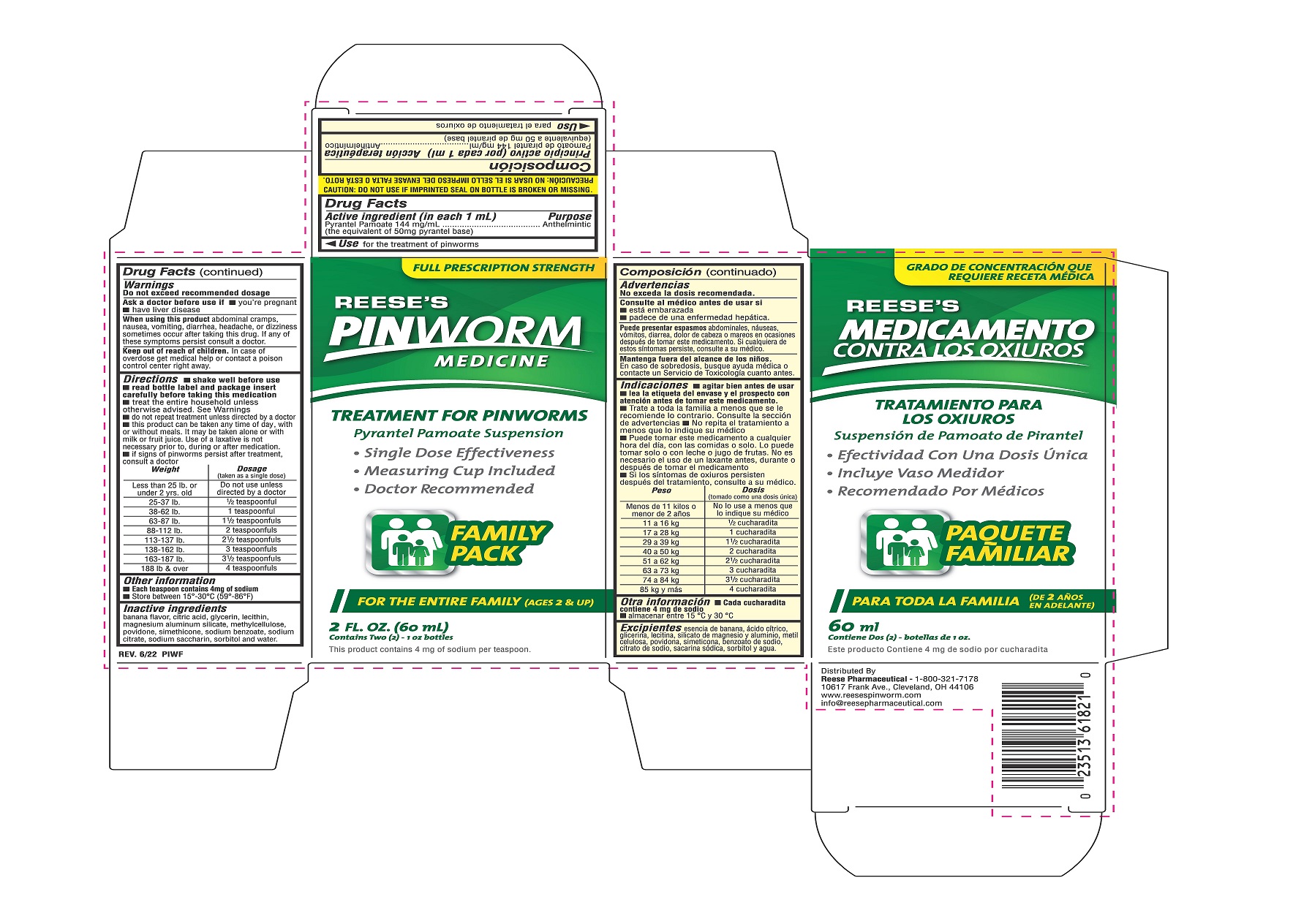
Label: PINWORM MEDICINE- pyrantel pamoate suspension
- NDC Code(s): 10956-778-01, 10956-778-02
- Packager: REESE PHARMACEUTICAL CO.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- WHEN USING
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions
- shake well before use
- read bottle label and package insert carefully before taking this medication
- treat the entire household unless otherwise advised
- do not repeat treatment unless directed by a doctor
- this product can be taken any time of day, with or without meals. It may be taken alone or with milk or fruit juice. Use of a laxative is not necessary prior to, during or after medication
- it signs of pinworms persist after treatment, consult a doctor
Weight Dosage (taken as a single dose) Less than 25 lb. or under 2 yrs.old Do not use unless directed by a doctor 25-37 lb. 1/2 teaspoonful 38-62 lb. 1 teaspoonful 63-87 lb. 1 1/2 teaspoonfuls 88-112 lb. 2 teaspoonfuls 113-137 lb. 2 1/2 teaspoonfuls 138-162 lb. 3 teaspoonfuls 163-187 lb. 3 1/2 teaspoonfuls 188 lb. and over 4 teaspoonfuls - INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PINWORM MEDICINE
pyrantel pamoate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10956-778 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRANTEL PAMOATE (UNII: 81BK194Z5M) (PYRANTEL - UNII:4QIH0N49E7) PYRANTEL 144 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLCELLULOSE (100 CPS) (UNII: 4GFU244C4J) POVIDONE (UNII: FZ989GH94E) DIMETHICONE (UNII: 92RU3N3Y1O) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor BANANA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10956-778-01 1 in 1 CARTON 09/28/2022 1 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:10956-778-02 2 in 1 CARTON 09/28/2022 2 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M024 09/28/2022 Labeler - REESE PHARMACEUTICAL CO. (004172052) Registrant - REESE PHARMACEUTICAL CO. (004172052) Establishment Name Address ID/FEI Business Operations LGM Pharma Solutions, LLC 117549198 manufacture(10956-778)