Label: LIDOCAINE HYDROCHLORIDE AND DEXTROSE- lidocaine hydrochloride injection, solution
- NDC Code(s): 0338-0409-02, 0338-0409-03, 0338-0411-02
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 7, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
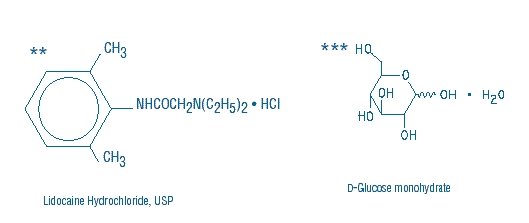
DESCRIPTIONLidocaine Hydrochloride and 5% Dextrose Injection, USP is a sterile, nonpyrogenic solution prepared from lidocaine hydrochloride and dextrose in water for injection. It contains no antimicrobial ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Lidocaine hydrochloride exerts an antiarrhythmic effect by increasing the electrical stimulation threshold of the ventricle during diastole. In usual therapeutic doses ...
-
INDICATIONS AND USAGELidocaine hydrochloride administered intravenously is specifically indicated in the acute management of (1) ventricular arrhythmias occurring during cardiac manipulations, such as cardiac surgery ...
-
CONTRAINDICATIONSHypersensitivity reactions, including anaphylactic reactions, have been reported with lidocaine. Lidocaine hydrochloride is contraindicated in patients with a history of hypersensitivity to local ...
-
WARNINGSConstant monitoring with an electrocardiograph is essential to the administration of lidocaine hydrochloride intravenously. Signs of excessive depression of cardiac conductivity, such as ...
-
PRECAUTIONSGeneral: If malignant hyperthermia develops, discontinue administration immediately and institute therapeutic countermeasures as clinically indicated. Lidocaine hydrochloride should not be ...
-
ADVERSE REACTIONSSystemic reactions of the following types have been reported: Nervous System Disorders: respiratory depression and arrest; unconsciousness; convulsions; tremors; twitching; vomiting; blurred or ...
-
OVERDOSAGESigns and symptoms of overdose may include: • Central nervous system effects, e.g., coma, loss of consciousness, CNS depression, seizure, tonic-clonic muscle jerks, tremor, nystagmus, tingling of ...
-
DOSAGE AND ADMINISTRATIONTherapy of ventricular arrhythmias is often initiated with a single IV bolus of 1.0 to 1.5 mg/kg at a rate of 25 to 50 mg/min. of lidocaine hydrochloride injection. Following acute treatment by ...
-
HOW SUPPLIEDLidocaine Hydrochloride and 5% Dextrose Injection, USP in VIAFLEX plastic container is available as follows: CodeSize (mL)NDCProduct Name - 2B0972 - 250 - 0338-0409-02 - Lidocaine ...
-
SPL UNCLASSIFIED SECTIONBaxter Healthcare Corporation - Deerfield, IL 60015 USA - Printed in USA - 07-19-76-975 - Rev. February 2017 - BAXTER, VIAFLEX, and PL 146 are trademarks of Baxter International Inc.
-
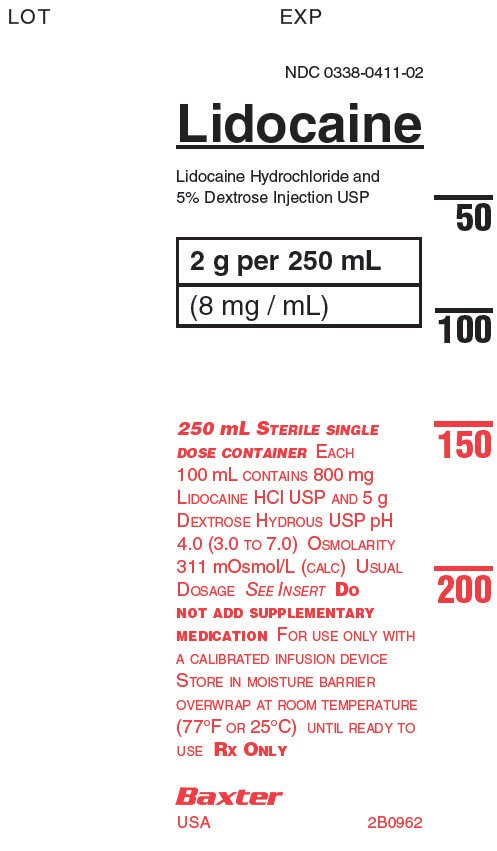
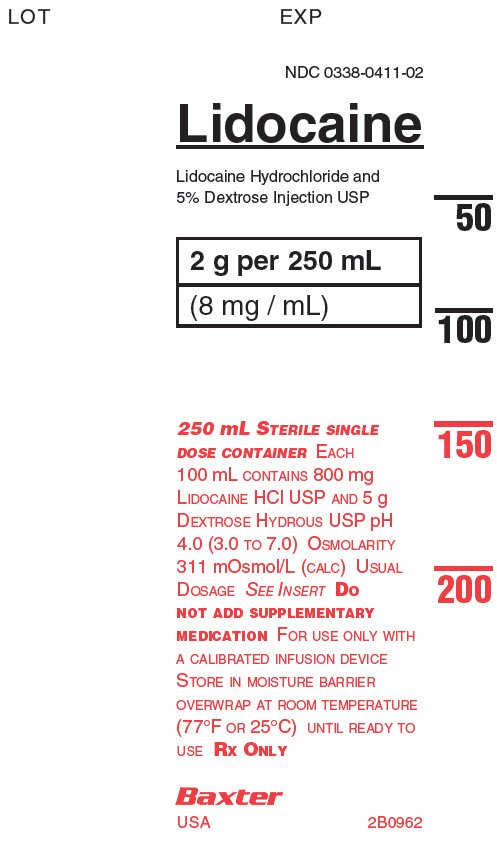
PACKAGE LABEL - PRINCIPAL DISPLAY PANELContainer - Container - LOT - EXP - NDC 0338-0411-02 - Lidocaine - Lidocaine Hydrochloride and - 5% Dextrose Injection USP - 2g per 250 mL - (8 mg/mL) 250 mL STERILE SINGLE - DOSE CONTAINER EACH ...
-
INGREDIENTS AND APPEARANCEProduct Information