General
-
Prescribing clarithromycin extended-release tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to ...
General
Prescribing clarithromycin extended-release tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Clarithromycin is principally excreted via the liver and kidney. Clarithromycin may be administered without dosage adjustment to patients with hepatic impairment and normal renal function. However, in the presence of severe renal impairment with or without coexisting hepatic impairment, decreased dosage or prolonged dosing intervals may be appropriate.
Clarithromycin in combination with ranitidine bismuth citrate therapy is not recommended in patients with creatinine clearance less than 25 mL/min. (See DOSAGE AND ADMINISTRATION.)
Clarithromycin in combination with ranitidine bismuth citrate should not be used in patients with a history of acute porphyria.
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving clarithromycin therapy.
For information about precautions of other drugs indicated in combination with clarithromycin, refer to the PRECAUTIONS section of their package inserts.
Information to Patients
Patients should be counseled that antibacterial drugs including clarithromycin extended-release tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When clarithromycin extended-release tablet is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by clarithromycin extended-release tablets or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Clarithromycin may interact with some drugs; therefore patients should be advised to report to their doctor the use of any other medications.
Clarithromycin extended-release tablets should be taken with food.
Drug Interactions
Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations. Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range. In two studies in which theophylline was administered with clarithromycin (a theophylline sustained-release formulation was dosed at either 6.5 mg/kg or 12 mg/kg together with 250 or 500 mg q12h clarithromycin), the steady-state levels of C max, Cmin, and the area under the serum concentration time curve (AUC) of theophylline increased about 20%.
Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered.
When clarithromycin and terfenadine were coadministered, plasma concentrations of the active acid metabolite of terfenadine were threefold higher, on average, than the values observed when terfenadine was administered alone. The pharmacokinetics of clarithromycin and the 14-hydroxy-clarithromycin were not significantly affected by coadministration of terfenadine once clarithromycin reached steady-state conditions. Concomitant administration of clarithromycin with terfenadine is contraindicated. (See CONTRAINDICATIONS.)
Simultaneous oral administration of clarithromycin tablets and zidovudine to HIV-infected adult patients resulted in decreased steady-state zidovudine concentrations. When 500 mg of clarithromycin were administered twice daily, steady-state zidovudine AUC was reduced by a mean of 12% (n = 4). Individual values ranged from a decrease of 34% to an increase of 14%. Based on limited data in 24 patients, when clarithromycin tablets were administered two to four hours prior to oral zidovudine, the steady-state zidovudine Cmax was increased by approximately 2-fold, whereas the AUC was unaffected.
Simultaneous administration of clarithromycin tablets and didanosine to 12 HIV-infected adult patients resulted in no statistically significant change in didanosine pharmacokinetics.
Concomitant administration of fluconazole 200 mg daily and clarithromycin 500 mg twice daily to 21 healthy volunteers led to increases in the mean steady-state clarithromycin Cmin and AUC of 33% and 18%, respectively. Steady-state concentrations of 14-OH clarithromycin were not significantly affected by concomitant administration of fluconazole.
Concomitant administration of clarithromycin and ritonavir (n = 22) resulted in a 77% increase in clarithromycin AUC and a 100% decrease in the AUC of 14-OH clarithromycin. Clarithromycin may be administered without dosage adjustment to patients with normal renal function taking ritonavir. However, for patients with renal impairment, the following dosage adjustments should be considered. For patients with CL CR 30 to 60 mL/min, the dose of clarithromycin should be reduced by 50%. For patients with CLCR < 30 mL/min, the dose of clarithromycin should be decreased by 75%.
Spontaneous reports in the postmarketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously.
Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have also been reported in postmarketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Serum digoxin concentrations should be carefully monitored while patients are receiving digoxin and clarithromycin simultaneously.
Colchicine is a substrate for both CYP3A and the efflux transporter, Pglycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. When clarithromycin and colchicine are administered together, inhibition of Pgp and/or CYP3A by clarithromycin may lead to increased exposure to colchicine. Patients should be monitored for clinical symptoms of colchicine toxicity. (See WARNINGS.)
Erythromycin and clarithromycin are substrates and inhibitors of the 3A isoform subfamily of the cytochrome P450 enzyme system (CYP3A). Coadministration of erythromycin or clarithromycin and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both the therapeutic and adverse effects of the concomitant drug. Dosage adjustments may be considered, and when possible, serum concentrations of drugs primarily metabolized by CYP3A should be monitored closely in patients concurrently receiving clarithromycin or erythromycin.
The following are examples of some clinically significant CYP3A based drug interactions. Interactions with other drugs metabolized by the CYP3A isoform are also possible. Increased serum concentrations of carbamazepine and the active acid metabolite of terfenadine were observed in clinical trials with clarithromycin.
The following CYP3A based drug interactions have been observed with erythromycin products and/or with clarithromycin in postmarketing experience:
Antiarrhythmics
There have been postmarketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during coadministration of clarithromycin with these drugs. Serum concentrations of these medications should also be monitored.
Ergotamine/Dihydroergotamine
Post-marketing reports indicate that coadministration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system. Concomitant administration of clarithromycin with ergotamine or dihydroergotamine is contraindicated (see CONTRAINDICATIONS).
Triazolobenziodidiazepines (Such as Triazolam and Alprazolam) and Related Benzodiazepines (Such as Midazolam)
Erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines. There have been post-marketing reports of drug interactions and CNS effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam.
HMG-CoA Reductase Inhibitors
As with other macrolides, clarithromycin has been reported to increase concentrations of HMG-CoA reductase inhibitors (e.g., lovastatin and simvastatin). Rare reports of rhabdomyolysis have been reported in patients taking these drugs concomitantly.
Sildenafil (Viagra)
Erythromycin has been reported to increase the systemic exposure (AUC) of sildenafil. A similar interaction may occur with clarithromycin; reduction of sildenafil dosage should be considered. (See Viagra package insert.)
There have been spontaneous or published reports of CYP3A based interactions of erythromycin and/or clarithromycin with cyclosporine, carbamazepine, tacrolimus, alfentanil, disopyramide, rifabutin, quinidine, methylprednisolone, cilostazol, and bromocriptine.
Concomitant administration of clarithromycin with cisapride, pimozide, astemizole, or terfenadine is contraindicated (see CONTRAINDICATIONS.)
In addition, there have been reports of interactions of erythromycin or clarithromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate.
Carcinogenesis, Mutagenesis, Impairment of Fertility
The following in vitro mutagenicity tests have been conducted with clarithromycin:
Salmonella/Mammalian Microsomes Test
Bacterial Induced Mutation Frequency Test
In VitroChromosome Aberration Test
Rat Hepatocyte DNA Synthesis Assay
Mouse Lymphoma Assay
Mouse Dominant Lethal Study
Mouse Micronucleus Test
All tests had negative results except the In Vitro Chromosome Aberration Test which was weakly positive in one test and negative in another.
In addition, a Bacterial Reverse-Mutation Test (Ames Test) has been performed on clarithromycin metabolites with negative results.
Fertility and reproduction studies have shown that daily doses of up to 160 mg/kg/day (1.3 times the recommended maximum human dose based on mg/m 2) to male and female rats caused no adverse effects on the estrous cycle, fertility, parturition, or number and viability of offspring. Plasma levels in rats after 150 mg/kg/day were 2 times the human serum levels.
In the 150 mg/kg/day monkey studies, plasma levels were 3 times the human serum levels. When given orally at 150 mg/kg/day (2.4 times the recommended maximum human dose based on mg/m 2), clarithromycin was shown to produce embryonic loss in monkeys. This effect has been attributed to marked maternal toxicity of the drug at this high dose.
In rabbits, in utero fetal loss occurred at an intravenous dose of 33 mg/m2, which is 17 times less than the maximum proposed human oral daily dose of 618 mg/m2.
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of clarithromycin.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Four teratogenicity studies in rats (three with oral doses and one with intravenous doses up to 160 mg/kg/day administered during the period of major organogenesis) and two in rabbits at oral doses up to 125 mg/kg/day (approximately 2 times the recommended maximum human dose based on mg/m 2) or intravenous doses of 30 mg/kg/day administered during gestation days 6 to 18 failed to demonstrate any teratogenicity from clarithromycin. Two additional oral studies in a different rat strain at similar doses and similar conditions demonstrated a low incidence of cardiovascular anomalies at doses of 150 mg/kg/day administered during gestation days 6 to 15. Plasma levels after 150 mg/kg/day were 2 times the human serum levels. Four studies in mice revealed a variable incidence of cleft palate following oral doses of 1000 mg/kg/day (2 and 4 times the recommended maximum human dose based on mg/m2, respectively) during gestation days 6 to 15. Cleft palate was also seen at 500 mg/kg/day. The 1000 mg/kg/day exposure resulted in plasma levels 17 times the human serum levels. In monkeys, an oral dose of 70 mg/kg/day (an approximate equidose of the recommended maximum human dose based on mg/m2) produced fetal growth retardation at plasma levels that were 2 times the human serum levels.
There are no adequate and well-controlled studies in pregnant women. Clarithromycin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. (See WARNINGS.)
Nursing Mothers
It is not known whether clarithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when clarithromycin is administered to a nursing woman. It is known that clarithromycin is excreted in the milk of lactating animals and that other drugs of this class are excreted in human milk. Preweaned rats, exposed indirectly via consumption of milk from dams treated with 150 mg/kg/day for 3 weeks, were not adversely affected, despite data indicating higher drug levels in milk than in plasma.
Pediatric Use
Safety and effectiveness of clarithromycin in pediatric patients under 6 months of age have not been established. The safety of clarithromycin has not been studied in MAC patients under the age of 20 months. Neonatal and juvenile animals tolerated clarithromycin in a manner similar to adult animals. Young animals were slightly more intolerant to acute overdosage and to subtle reductions in erythrocytes, platelets and leukocytes but were less sensitive to toxicity in the liver, kidney, thymus, and genitalia.
Geriatric Use
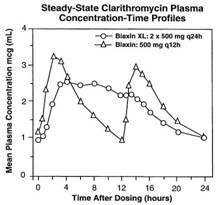
In a steady-state study in which healthy elderly subjects (age 65 to 81 years old) were given 500 mg every 12 hours, the maximum serum concentrations and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults. These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse events when compared to younger patients. Dosage adjustment should be considered in elderly patients with severe renal impairment.
Close